"... - How many parrots fit in you, such an increase.
- Really needed! I will not swallow so many parrots! ... "
From m / f "38 parrots"
In accordance with the international rules of C (international system of units of measurement), the number of thermal energy or the amount of heat is measured in Joules [J], there are also multiple kilodzhoule units [kJ] \u003d 1000 J., Meghadzhoule [MJ] \u003d 1 000 000 J, Gigajoule [ GJ] \u003d 1 000 000 000 J., etc. This unit of measuring thermal energy is the main international unit and is most often used when conducting scientific and scientific and technical calculations.
However, all of us know or at least once heard another unit of measurement of the amount of heat (or simply heat) is a calorie, as well as kilocaloria, megaklorine and gigakloria, which means Cilo, Giga and Mega consoles, see an example with Joules above. In our country, historically developed in such a way that when calculating heating tariffs, be it the heating of electricity, gas or pellet boilers it is customary to consider the cost of exactly one gigaklorine of thermal energy.
So what is gigaklorine, kilowatt, kilowatt * an hour or kilowatt / hour and jouli and how are they related to each other?, You will learn in this article.
So, the main unit of thermal energy is, as already mentioned, Joule. But before talking about the units of measurement, it is necessary in principle on the household level to clarify what kind of thermal energy is and how and what to measure it.
We know all of your childhood to warm up (get heat) you need to set up something, so we all burned fires, traditional fuel for the fire is a firewood. Thus, obviously, when burning fuel (any: firewood, coal, pellets, natural gas, diesel fuel), thermal energy (heat) is distinguished. But to heat, for example, various volumes of water requires a different amount of firewood (or other fuel). It is clear that for heating two liters of water, several fives in the fire, and to prepare half a soup to the entire camp, you need to stock up with several knitters of firewood. In order not to measure such strict technical values \u200b\u200bas the amount of heat and heat combustion of fuels with fuels of firewood and shoulders with soup, heat engineers decided to make clarity and order and agreed to invent a unit of the amount of heat. So that this unit was everywhere the same determined it: to heat one kilogram of water for one degree under normal conditions (atmospheric pressure), 4,190 calories are required, or 4,19 kokaloria, therefore, to heat one gram of water will be quite a thousand times less heat - 4,19 calories.
Calorie is associated with the international unit of thermal energy - Joule the following ratio:
1 calorie \u003d 4.19 Joule.
Thus, for heating 1 gram of water for one degree, 4.19 joule of thermal energy will be required, and for heating one kilogram of water 4 190 joule heat.
In the technique, along with a unit of measurement of thermal (and other) energy, there is a power unit and, in accordance with the international system (C), this is Watt. The concept of power also applies to heating devices. If the heating device is able to give 1 second to 1 joule thermal energy in 1 second, then its power is 1 watt. Power, this is the ability to produce (create) a certain amount of energy (in our case of thermal energy) per unit of time. Let's return to our example with water to heat one kilogram (or one liter, in the case of a kilogram water is equal to liter) of water for one degree Celsius (or Celvin, without a difference), we will need 1 cywlolarium power or 4,130 J. heat energy. To heat one kilogram of water in 1 second time for 1 cruds, we need the device next power:
4190 J. / 1 \u200b\u200bs. \u003d 4 190 W. or 4.19 kW.
If we want to heat our kilogram of water for 25 degrees for the same second, we will need power every twenty five times more than.
4,19 * 25 \u003d 104.75 kW.
Thus, it can be concluded that a pellet boiler with a capacity of 104.75 kW. Heats 1 liter of water by 25 degrees in one second.
Since we got to Watt and Kilowatt, you should also fold a word about them. As Watt has already been said - this is a power unit, including the heat of the boiler, but in addition to pellet boilers and gas boilers, humanity is familiar and electrocotels, the capacity of which is measured, of course, in the same kilowatts and they consume not pellets and not gas, And electricity, the amount of which is measured in kilowatt hours. Proper spelling of the unit of energy kilowatt * Hour (it is, kilowatt multiplied for an hour, not divided), recording kW / hour - is an error!
In electrocotes, electrical energy is transformed into thermal (so-called, Jowlevo heat), and if the boiler consumes 1 kW * hour of electricity, how much did it work out heat? To respond to this simple question, you need to perform a simple calculation.
We transform kilowatts to Kilodzhoule / seconds (kilodzhoule per second), and hours per second: in one hour 3,600 seconds, we get:
1 kW * hour \u003d [1 kJ / s] * 3600 c. \u003d 1 000 J * 3600 C \u003d 3,600,000 Joule or 3.6 MJ.
So,
1 kW * hour \u003d 3.6 MJ.
In turn, 3.6 mJ / 4.19 \u003d 0.859 μal \u003d 859 kcal \u003d 859,000 feces. Energy (thermal).
Now let's turn to the gigakloria whose price on various types of fuel is like to consider heat engineering.
1 Gcal \u003d 1 000 000 000 kal.
1 000 000 000 kal. \u003d 4.19 * 1 000 000 000 \u003d 4 190 000 000 J. \u003d 4 190 MJ. \u003d 4.19 GJ.
Or knowing that 1 kW * hour \u003d 3.6 MJ, we recalculate 1 gigaklora at kilowatt * Watch:
1 Gcal \u003d 4190 MJ / 3.6 MJ \u003d 1 163 kW * hours!
If you have read this article, you decide to consult with a specialist of our company on any issue related to heat supply, then you Here!
Source: Teplo-en.ru.
(or heat transfer).
Specific heat capacity of the substance.
Heat capacity - This is the amount of heat absorbed by the body when heated by 1 degree.
The heat capacity of the body is indicated by the title Latin letter FROM.
What depends on the heat capacity of the body? First of all, from its mass. It is clear that for heating, for example, 1 kilogram of water will need more heat than for heating 200 grams.
And from the kind of substance? We do experience. Take two identical vessels and, in one of them, water weighing 400, and in the other - vegetable oil weighing 400 g, we begin to heat them with the same burner. Watching the testimony of thermometers, we will see that the oil heats up quickly. To heat the water and oil to the same temperature, water should be heated longer. But the longer we heated the water, the greater the amount of heat it gets from the burner.
Thus, for heating the same mass of different substances up to the same temperature, a different amount of heat is required. The amount of heat required to heat the body and, therefore, its heat capacity depend on the kind of substance from which this body consists.
For example, to increase by 1 ° C water temperature weighing 1 kg, the amount of heat is required, equal to 4200 J, and for heating by 1 ° C of the same mass of sunflower oil, the amount of heat equal to 1700 J.
The physical value showing how much heat is required for heating 1 kg of substance per 1 ºС, called specific heat This substance.
Each substance has its own specific heat, which is indicated by the Latin letter C and is measured in Joules per kilogram-degree (J / (kg · ° C)).
The specific heat capacity of the same substance in different aggregate states (solid, liquid and gaseous) is different. For example, the specific heat capacity of water is 4200 J / (kg · ºС), and the specific heat capacity of ice is 2100 J / (kg · ° C); Aluminum in a solid state has a specific heat capacity equal to 920 J / (kg - ° C), and in liquid - 1080 J / (kg - ° C).
Note that water has a very greater specific heat capacity. Therefore, water in the seas and oceans, heating in summer, absorbs a large amount of heat from the air. Due to this, in those places that are located near the large water bodies, the summer is not so hot, both in places removed from the water.
Calculation of the amount of heat required to heat the body or the cooling allocated by it.
It is clear from the above that the amount of heat required for heating the body depends on the kind of substance from which the body consists (i.e. its specific heat), and from body weight. It is also clear that the amount of warmth depends on how much degrees we are going to increase body temperature.
So, in order to determine the amount of heat required for heating the body or the cooling allocated by it during cooling, the specific heat capacity of the body is multiplied by its mass and the difference between its finite and initial temperatures:
Q. = cm. (t. 2 - t. 1 ) ,
where Q. - quantity of heat, c. - specific heat, m. - body mass , t. 1 - initial pace, t. 2 - Finite temperature.
When heating the body t 2\u003e t. 1 And, therefore, Q. > 0 . When cooling the body t 2< t. 1 And, therefore, Q.< 0 .
In case the heat capacity of the whole body is known FROM, Q. Determined by the formula:
Q \u003d C (T 2 - t. 1 ) .
By definition, calorie is the amount of heat that is required to heat a single cubic centimeter of water to 1 degree Celsius. Gigaklorine used to measure thermal energy in thermal power and utilities is a billion calories. In 1 meter, 100 centimeters, therefore, in one cubic meter - 100 x 100 x 100 \u003d 1000000 centimeters. So to heat the water cube on
1 degree, it will take a million calorie or 0.001 Gcal.
In my city, the heating price is $ 1132,22 / GKAL, and the price of hot water - 71,65 rubles / cubic meter, the price of cold water is 16,77 rubles / cubic meters.
How many gkal is spent to warm 1 cube of water?
I think so
s x 1132,22 \u003d 71.65 - 16.77 and thus solving the equations to find out what is equal to S (Gcal), that is, is 0.0484711452 Gcal
I doubt something, in my opinion, I will not decide
ANSWER:
I do not find errors in your calculation.
Naturally, the cost of wastewater (drainage) should not be present in the above tariffs.
The approximate calculation in Izhevsk on old standards looks like this:
0.19 Gcal per person per month (this rate is now canceled, but the other is not, for example, it is suitable) / 3.6 cubic meters. per person per month (the rate of consumption of hot water) \u003d 0.05278 Gcal per 1 cubic meters. (So \u200b\u200bmuch need heat for heating 1 cubic meters. cold water to the normative temperature of hot water, which, remind, is 60 degrees. c).
For a more accurate calculation of the amount of thermal energy to heating the water by direct method on the basis of physical quantities (and not back through the approved tariffs for the DHW) - I recommend to use template for calculating tariff for hot water (REC). The calculation formula, among other things, uses the temperature of cold water in summer and winter (heating) periods, the duration of these periods.
Tags: gigakloria, hot water
- We pay for the GWS services, the temperature is significantly lower than the standard. What to do?
- Lastly established by the rules The shutdown term of the DHW is not illegal - the decision of the Supreme Court of the Russian Federation (2017)
- Initiative to establish more fair tariffs and methods of hot water consumption
- On the procedure for recalculating the size of the charge for heating and DHW during shutdowns - clarification of Rospotrebnadzor for UR
- On accounting of the coolant in a closed heat supply system - a letter of the Ministry of Internal Affairs of the Russian Federation of 31.03.2015 No. 9116-od / 04
- UR - on reducing the charge for heating and DHW - Letter of the Ministry of Energy of the UR of 08/17/2015 №11-10 / 5661
- What is the normative term for the verification of a general-friendly metering of heating and DHW?
- Dirty hot water from under the tap. Where to contact?
- Can the water meter in the apartment put it for the entire entrance? How to pay? Indications for the month - 42 cubic meters
- The procedure for maintaining a separate accounting of costs in the field of water supply and drainage - Order of the Ministry of Instruction of the Russian Federation of January 25, 2014 №22 /
- fee for water and electricity in the apartment without accommodation
- calculation of heat in OTP 1/12
- Power supply
- Huge payments for the room in the hostel (17.3 sq.m.)
| Comments: (11) | |
| Hint: Share a link in social networks, if you want to get more answers / comments! | |
730. Why is water used for cooling some mechanisms?
Water has a large specific heat capacity, which contributes to a good heat removal from the mechanism.
731. In which case, you need to spend more energy: for heating at 1 ° C of one liter of water or for heating at 1 ° C. one hundred grams of water?
To heat the liter of water, as the larger the mass, the more energy you need.
732. Melchive and silver plugs of the same mass lowered hot water. Do they get the same water to get the same water?
Melchior fork will receive more warmth, because the specific heat capacity of the Melchior is greater than silver.
733. At a piece of lead and on a piece of cast iron, the same mass was hit by a sledgehammer. Which piece was harder?
The lead heats up stronger, because its specific heat capacity is less than cast iron, and for heating lead is needed less energy.
734. In one flask there is water, in the other - kerosene of the same mass and temperature. Each flask was thrown at the same heated iron cube. What is heated to a higher temperature - water or kerosene?
Kerosene.
735. Why in the cities on the seaside of temperature fluctuations in winter and summer less cutting than in the cities located in the depths of the mainland?
Water heats up and cools slower than air. In winter, it cools and moves the warm mass of air to the land, making the climate on the shore warmer.
736. The specific heating capacity of aluminum is 920 J / kg ° C. What does this mean?
This means that for heating 1 kg of aluminum at 1 ° C, it is necessary to spend 920 J.
737. Aluminum and copper bars of the same mass of 1 kg are cooled at 1 ° C. How much will the internal energy change each bar? Which bar does it change more and how much?
738. What amount of heat is necessary to heat the kilogram iron billet to 45 ° C?
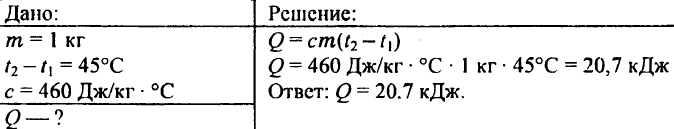
739. What amount of warmth is required to heat 0.25 kg of water from 30 ° C to 50 ° C?
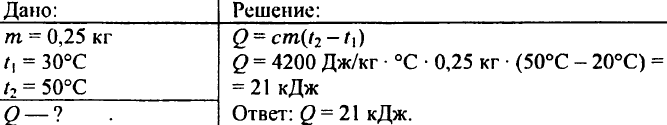
740. How will the internal energy change two liters of water when heated at 5 ° C?

741. What amount of heat is necessary for heating 5 g of water from 20 ° C to 30 ° C?

742. What amount of heat is necessary to heat the aluminum ball with a mass of 0.03 kg at 72 ° C?

743. Calculate the amount of heat required for heating 15 kg of copper at 80 ° C.

744. Calculate the amount of heat required for heating 5 kg of copper from 10 ° C to 200 ° C.

745. What amount of heat is required for heating 0.2 kg of water from 15 ° C to 20 ° C?

746. Water weighing 0.3 kg cooled at 20 ° C. How much did the internal energy of water decreased?

747. What amount of heat need to be 0.4 kg of water at a temperature of 20 ° C heat to a temperature of 30 ° C?

748. What amount of heat is spent on heating 2.5 kg of water at 20 ° C?

749. What amount of heat released when cooled 250 g of water from 90 ° C to 40 ° C?

750. What amount of heat will need to heat at 1 ° C 0.015 l?
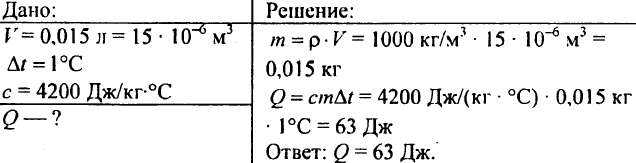
751. Calculate the amount of heat required to heat the 300 m3 pond at 10 ° C?

752. What amount of heat should I know 1 kg of water to increase its temperature from 30 ° C to 40 ° C?

753. The water of 10 L cooled from a temperature of 100 ° C to a temperature of 40 ° C. What amount of heat mediated at the same time?

754. Calculate the amount of heat required for heating 1 m3 of sand at 60 ° C.

755. Air volume is 60 m3, specific heat capacity of 1000 J / kg ° C, air density is 1.29 kg / m3. What amount of heat is needed to heat it at 22 ° C?

756. Water heated at 10 ° C, spending 4.20 103 J heat. Determine the amount of water.
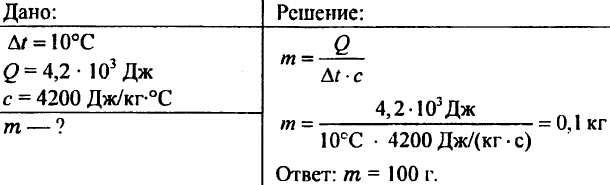
757. Water weighing 0.5 kg was reported to 20.95 kJ heat. What was the water temperature, if the initial water temperature was 20 ° C?

758. In a copper pan weighing 2.5 kg, 8 kg of water at 10 ° C. What amount of heat is necessary for water in a saucepan to boil?
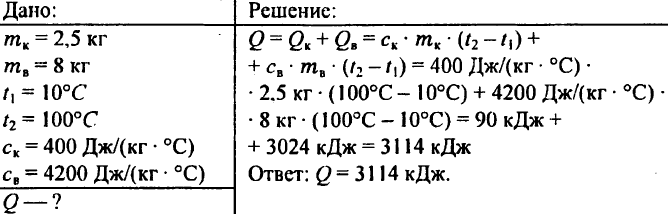
759. A liter of water at a temperature of 15 ° C is poured into a copper bucket weighing 300 g. What amount of warmth is necessary to heat the water in the bucket at 85 ° C?
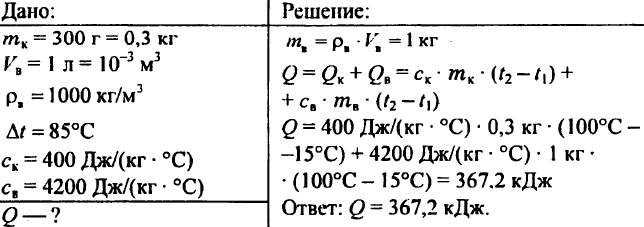
760. A piece of heated granite weighing 3 kg is placed in water. Granite transmits 12.6 kJ heat water, cooling at 10 ° C. What is the specific heat capacity of the stone?

761. To 5 kg of water at 12 ° C, the hot water was valued at 50 ° C, having obtained a mixture with a temperature of 30 ° C. How much water fastened?

762. In 3 liters of water at 60 ° C, the water was filled at 20 ° C, having obtained water at 40 ° C. How much water fastened?

763. What will be the temperature of the mixture, if you mix 600 g of water at 80 ° C with 200 g of water at 20 ° C?

764. A liter of water at 90 ° C was poured into water at 10 ° C, and the temperature of the water was 60 ° C. How much cold water was?

765. Determine how much it is necessary to pour hot water into a vessel, heated to 60 ° C, if 20 liters of cold water are already in the vessel at 15 ° C; The temperature of the mixture should be 40 ° C.
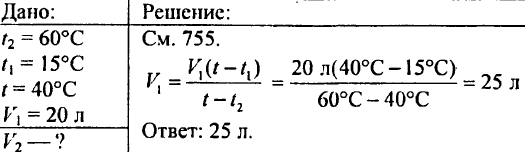
766. Determine how much heat is required for heating 425 g of water at 20 ° C.

767. How much degrees are 5 kg of water, if the water get 167.2 kJ?

768. How much heat is needed so that M of water grams at a temperature T1, heat to temperature T2?
769. In the calorimeter, 2 kg of water is poured at a temperature of 15 ° C. To which temperature is heated by the water of the calorimeter, if it is lowered into it a brass girc of 500 g, heated to 100 ° C? Specific heat capacity of brass 0.37 kJ / (kg ° C).

770. There are the same volume of slices of copper, tin and aluminum. Which of these pieces has the greatest and what lowest heat capacity?

771. The calorimeter was poured 450 g of water, the temperature of which is 20 ° C. When 200 g of iron sawders heated to 100 ° C were loaded into this water, the water temperature was 24 ° C. Determine the specific heat capacity of sawdust.

772. The copper calorimeter weighing 100 g accommodates 738 g of water, the temperature of which is 15 ° C. This calorimeter was lowered 200 g of copper at a temperature of 100 ° C, after which the calorimeter temperature rose to 17 ° C. What is the specific heat capacity of copper?

773. The steel ball weigh 10 g is removed from the furnace and lowered into water with a temperature of 10 ° C. The water temperature rose to 25 ° C. What was the temperature of the ball in the furnace, if the mass of water is 50 g? The specific heat capacity of the steel is 0.5 kJ / (kg ° C).
776. Water weighing 0.95 g at a temperature of 80 ° C was mixed with water weighing 0.15 g at a temperature of 15 ° C. Determine the temperature of the mixture. 779. Steel cutter weighing 2 kg was heated to a temperature of 800 ° C and then omitted to a vessel containing 15 liters of water at a temperature of 10 ° C. What temperature will the water be heated in the vessel?
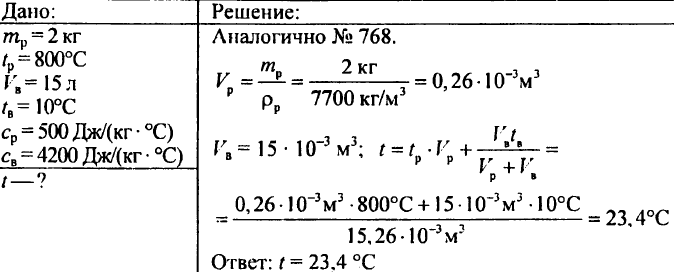
(Note. To solve this problem, it is necessary to make an equation in which the unknown adopt the desired water temperature in the vessel after lowering the cutter.)
780. What temperature is water, if you mix 0.02 kg of water at 15 ° C, 0.03 kg of water at 25 ° C and 0.01 kg of water at 60 ° C?

781. For heating a well-ventilated class, the amount of heat is 4.19 MJ per hour. Water enters the heating radiators at 80 ° C, and comes out of them at 72 ° C. How much water should be served every hour in radiators?

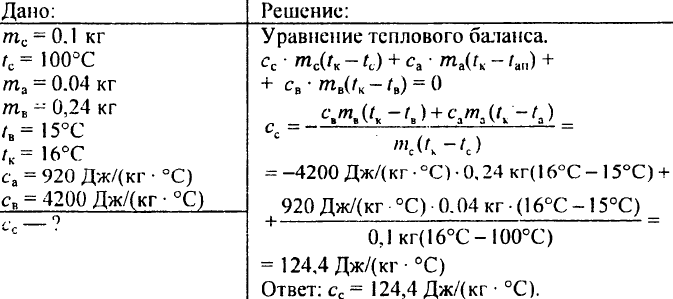
782. Lead weighing 0.1 kg at a temperature of 100 ° C was immersed in an aluminum calorimeter weighing 0.04 kg, containing 0.24 kg of water at 15 ° C. After that, a temperature of 16 ° C was installed in the calorimeter. What is the specific lead heat capacity?
Humanity knows some types of energy - mechanical energy (kinetic and potential), internal energy (thermal), field energy (gravitational, electromagnetic and nuclear), chemical. Separately, it is worth highlighting the energy of the explosion, ...
Vacuum energy and even existing only in theory - dark energy. In this article, first in the heading "Heat Engineering", I will try in a simple and accessible language using a practical example, to tell about the most important form of energy in the life of people - about thermal energy and about boring it in time thermal power.
A few words to understand the place of heat engineering, as the section of science on obtaining, transferring and using thermal energy. Modern heat engineering has been separated from the general thermodynamics, which in turn is one of the sections of physics. Thermodynamics is a literally "warm" plus "force". Thus, thermodynamics is a science of "change in temperature" of the system.
Impact on the outside system, in which its internal energy changes, may be the result of heat exchange. Thermal energywhich is purchased or lost by the system as a result of such interaction with the environment, is called amount of warmth And measured in the SI system in Joules.
If you are not an engineer-heat engineer, and do not every day do heat engineering issues, then you, facing them, sometimes without experience it is very difficult to figure them out. It is difficult without the presence of experience to present even the dimension of the required values \u200b\u200bof the amount of heat and thermal power. How many Joule energy is necessary to heat 1000 meters of cubic air from temperature -37˚С to + 18 ° C? .. What is the power of the heat source, to do it in 1 hour? .. On these not the most difficult questions are able to answer today. "Not all engineers. Sometimes experts even remember the formula, but only units can apply them in practice!
After reading this article to the end, you can easily solve real production and household problems associated with heating and cooling of various materials. Understanding the physical essence of heat transfer processes and knowledge of simple basic formulas is the main blocks in the foundation of knowledge on heat engineering!
The amount of heat in various physical processes.
Most of the known substances can be in solid, liquid, gaseous or plasma states at different temperatures and pressure. Transition from one aggregate state to another happens at a constant temperature (provided that pressure and other environmental parameters do not change) and is accompanied by the absorption or separation of thermal energy. Despite the fact that in the Universe, 99% of the substance is in a state of plasma, we will not consider this aggregate in this article.
Consider the schedule presented in the figure. It depicts the dependence of the temperature of the substance T. From the amount of warmth Q. submitted to a certain closed system containing a certain mass of some particular substance.

1. Solid T1. , heated to temperature TPL , spending on this process the amount of heat equal Q1. .
2. Next begins the melting process, which occurs at a constant temperature. TPL (melting point). To melting the entire mass of the solid body, it is necessary to spend the heat in the amount of thermal energy Q2. - Q1. .
3. Next, the liquid, obtained as a result of melting solid, heated to the boiling point (gas formation) TKP , spending on this amount of heat equal Q3.-Q2. .
4. Now at unchanged boiling point TKP Liquid boils and evaporates, turning into gas. To switch the entire mass of fluid to gas, it is necessary to spend thermal energy in the amount Q4.-Q3..
5. At the last stage, gas is heated on temperature TKP To some temperature T2. . In this case, the cost of the amount of heat will be Q5.-Q4. . (If they are rapid gas to the ionization temperature, then the gas will turn into a plasma.)
Thus, heating source solid from temperature T1. to temperature T2. We spent thermal energy in quantity Q5. , translating the substance through three aggregate states.
Moving in the opposite direction, we will assign from the substance the same amount of heat Q5.passing the steps of condensation, crystallization and cooling from temperature T2. to temperature T1. . Of course, we consider a closed system without energy loss to the external environment.
Note that it is possible to transition from a solid state into a gaseous state, bypassing the liquid phase. Such a process is referred to as the sublimation, and the reverse process to it - desublimation.
So, we understood that the transition processes between the aggregate states of the substance are characterized by energy consumption at a constant temperature. When heating the substance located in one unchanged aggregate state, the temperature rises and thermal energy is also consumed.
The main formulas of heat transfer.
Formulas are very simple.
Quantity of heat Q. In J, it is calculated by the formulas:
1. From the consumption of heat, that is, from the load side:
1.1. When heated (cooling):
Q. = m. * c. * (T2 -T1)
m. – mass substance in kg
from -specific heat capacity of the substance in J / (kg * k)
1.2. When melting (freezing):
Q. = m. * λ
λ – specific heat melting and crystallization of matter in j / kg
1.3. When boiling, evaporation (condensation):
Q. = m. * r.
r. – specific heat of gas formation and condensation of matter in j / kg
2. From the heat of heat, that is, from the side of the source:
2.1. When combustion of fuel:
Q. = m. * q.
q. – specific heat combustion of fuel in j / kg
2.2. When transforming electricity into thermal energy (the law of Joule - Lenza):
Q \u003d T * I \u200b\u200b* U \u003d T * R * i ^ 2 \u003d (t / R)* U ^ 2
t. – time in S.
I. – active value of current in a
U. – the active value of the voltage in
R. – load resistance in Ohm
We conclude - the amount of heat is directly proportional to the mass of the substance at all phase transformations and when heated is additionally directly in proportion to the temperature difference. Proportionality coefficients ( c. , λ , r. , q. ) For each substance, they have their own values \u200b\u200band are determined by the experimental way (taken from reference books).
Thermal power N. In W, this is the amount of heat transferred by the system for a certain time:
N \u003d Q / T
The faster we want to heat the body up to a certain temperature, the greater the power should be the source of thermal energy - everything is logical.
Calculation in Excel Application Tasks.
In life, it is often necessary to make a quick evaluation calculation to understand whether it makes sense to continue learning the topic, making the project and deployed accurate labor-intensive calculations. Making a calculation in a few minutes even with an accuracy of ± 30%, you can take an important managerial solution that will be 100 times cheaper and 1000 times more operational and as a result 100,000 times more efficient than performing an accurate calculation within a week, and then and month, a group of expensive specialists ...
Conditions of the problem:
To the premises of the workshop of the preparation of metal with dimensions of 24m x 15m x 7m bring from a warehouse on a metal-rolling street in the amount of 3T. On the metal there is an ice with a total weight of 20 kg. On Street -37˚С. How much heat is needed to heat the metal to + 18 ° C; Heat the ice, melt it and heat the water to + 18 ° C; Heat the entire volume of air indoors, assuming that before that, the heating was completely disabled? What power should have the heating system, if all of the above must be completed for 1 hour? (Very tough and almost no real conditions - especially concerning air!)
Calculation will be executed in the programMS Excel or in the programOoo Calc..
With the color formatting of cells and fonts, check out the page "".
Initial data:
1. The names of the substance write:
in the D3 cell: Steel
in cell E3: Ice
in cell F3: Ice / water
in cell G3: Water
in cell G3: Air
2. Process names We introduce:
in cells D4, E4, G4, G4: heat
in the F4 cell: melting
3. Specific heat capacity c. in j / (kg * k) we write for steel, ice, water and air, respectively
in the D5 cell: 460
in cell E5: 2110
in the cell G5: 4190
in cell H5: 1005
4. Specific warmth of melting ice λ in j / kg fit
in cell F6: 330000
5. Mass of substances m. in kg fit respectively for steel and ice
in the D7 cell: 3000
in cell E7: 20
Since when the ice turning into water, the mass does not change,
in cells F7 and G7: \u003d E7 =20
The mass of air we find the product of the room on the proportion
in the H7 cell: \u003d 24 * 15 * 7 * 1,23 =3100
6. Time processes t. in min write only once for steel
in the D8 cell: 60
The time for the heating of ice, its melting and heating of the resulting water is calculated from the condition that all these three processes should meet in the amount of the same time as the metal is applied to heating. Read respectively
in cell E8: \u003d E12 / (($ E $ 12 + $ F $ 12 + $ G $ 12) / d8) =9,7
in the cell F8: \u003d F12 / (($ E $ 12 + $ F $ 12 + $ G $ 12) / d8) =41,0
in the cell G8: \u003d G12 / (($ E $ 12 + $ F $ 12 + $ G $ 12) / d8) =9,4
Air should also warm up for the same allotted time, read
in the H8 cell: \u003d d8 =60,0
7. Initial temperature of all substances T.1 In C CO
in the D9 cell: -37
in cell E9: -37
in cell F9: 0
in the cell G9: 0
in the cell H9: -37
8. Finite temperature of all substances T.2 In C CO
in the D10 cell: 18
in cell E10: 0
in the F10 cell: 0
in the cell G10: 18
in cell H10: 18
I think questions according to p. 7 and paragraph 8 be unfinished.

Results of calculations:
9. Quantity of heat Q. In the KJ, which is necessary for each of the processes, we expect
for heating steel in the D12 cell: \u003d d7 * d5 * (D10-D9) / 1000 =75900
for ice heating in cell E12: \u003d E7 * E5 * (E10-E9) / 1000 = 1561
for melting ice in cell F12: \u003d F7 * F6 / 1000 = 6600
to heat the water in the cell G12: \u003d G7 * G5 * (G10-G9) / 1000 = 1508
for air heating in the H12 cell: \u003d H7 * H5 * (H10-H9) / 1000 = 171330
The total number of thermal energy required for all processes read
in the combined cell D13E13F13G13H13: \u003d sums (D12: H12) = 256900
In the cells D14, E14, F14, G14, H14, and the combined cell D15E15F15G15H15, the amount of heat is given in an arc unit of measurement - in Gcal (in gigakloria).
10. Thermal power N. In kW, the necessary for each of the processes is calculated
for heating steel in the cell D16: \u003d D12 / (D8 * 60) =21,083
for ice heating in cell E16: \u003d E12 / (E8 * 60) = 2,686
for melting ice in cell F16: \u003d F12 / (F8 * 60) = 2,686
to heat the water in the cell G16: \u003d G12 / (G8 * 60) = 2,686
for air heating in the cell H16: \u003d H12 / (H8 * 60) = 47,592
Total thermal power required to fulfill all processes during the time t. calculated
in the combined cell D17E17F17G17H17: \u003d D13 / (D8 * 60) = 71,361
In cells D18, E18, F18, G18, H18, and the combined cell D19E19F19G19H19, thermal power is given in the arc unit of measurement - in Gkal / hour.
This calculation in Excel is completed.
Conclusions:
Note that it is necessary to spend more than twice the air to heating the air than for heating the same mass of steel.
When water is heated, energy costs are twice as bigger than when the ice is heated. The melting process repeatedly consumes energy than the heating process (with a small temperature difference).
Water heating ten times spends more thermal energy than steel heating and four times more than air heating.
For receipt information about the release of new articles and for download program working files please subscribe to the announcements in the window located at the end of the article or in the top of the page.
After entering the address of your email and click on the "Receive Announcements" button DO NOT FORGETConfirm Subscription click on the link in a letter, which will immediately come to you on the specified mail (sometimes in the folder « Spam » )!
We remembered the concepts of "the amount of heat" and "thermal power", considered the fundamental formulas of heat transfer, disassembled a practical example. I hope my tongue was simple, understandable and interesting.
Waiting for questions and comments on the article!
ask Respectful work author download file After subscription on the announcements of articles.






