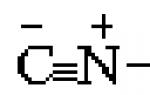There were frequent cases in the history of chemistry in which poisoning, injury or even death occurred not as a result of prolonged work with toxic substances, but as a result of one bad experience, usually accompanied by an explosion. Below is a far from complete list of such incidents.
AND the French chemist C.L. Berthollet (1748–1822) almost died while listening to the properties of the substance he discovered, later called berthollet's salt.
In one of the attempts to obtain potassium by heating a mixture of potassium hydroxide with powdered iron, the French scientists J.L. Gay-Lussac (1778-1850) and L.J. Tenard (1777-1857) almost lost their lives. To recover from his wounds, Gay-Lussac had to spend almost a month and a half in bed, his eyesight was temporarily lost. Thenar nearly died one more time in the chemical laboratory. In 1825, at a lecture, wishing to quench his thirst, he mistakenly drank liquid from a glass in which there was a solution of mercuric chloride (mercuric chloride HgCl 2, as you know, a strong poison). Only the timely taken antidote in the form of raw eggs saved his life.
The victim of another accident was the French chemist and physicist Pierre-Louis Dulong (1785-1838). In 1811, while studying nitrogen chloride, an explosion occurred in his laboratory, which severely concussed the scientist. Despite this, Dulong decided to continue researching the substance. In October 1812, a new explosion deprived him of an eye and disfigured his arm. Dulong's second eye also suffered. The scientist was only 27 years old at that time.
Serious poisoning as a result of working with hydrogen selenide was received in the spring of 1818 by the great Swedish chemist J.J. Berzelius (1779-1848).
On November 9, 1836, a sealed glass vessel with an arsenic compound exploded in the hands of the German chemist R.V.Bunsen (1811–1899), which almost led to the death of the scientist. A shard of glass hit Bunsen's right eye, blinding him forever. In addition, the scientist was poisoned.
A powerful explosion also occurred in the work of the French chemist S.A. Würz (1817–1884), when he heated a mixture of phosphorus trichloride with sodium in an open test tube. Numerous fragments seriously injured the scientist's face and hands. The glass also got into the eyes. The shards could not be removed immediately. Only over time, they began to gradually come out, and the surgeons had to apply all their skills to preserve Würz's vision.
The life of the future Nobel laureate German organic chemist A. Bayer (1835-1917) could have tragically ended in his youth. Working with methyldichloroarsine CH 3 AsCl 2, he was so badly poisoned that he fell to the floor of the laboratory, losing consciousness. Only emergency assistance to FA Kekule (1829–1896), who pulled the victim out into the fresh air, made it possible to avoid trouble. Bayer had to spend several days in bed. The skin on his face was red and severely inflamed.
Like Kekule, the German chemist A. Fischer saved his employee Y. Tafel from inevitable death after the latter was poisoned by acrolein vapors.
While working in Meyer's laboratory in Göttingen, in 1885, the famous Russian chemist ND Zelinsky (1861-1953) received a serious poisoning with 2,2 "-dichlorodiethyl sulfide ClCH 2 CH 2 –S – CH 2 CH 2 Cl. he had blisters on his hands, face and body. The scientist had to spend several months in hospital. The substance obtained by him was later used by the Germans in 1917 in the region of Ypres, after which it was named "mustard gas".
In 1884, L.Yu. Meyer (1830–1895), who loved to demonstrate the explosion of an air-acetylene mixture, was seriously injured in 1884. Once, during such a demonstration, an explosion occurred of such force that it destroyed all the equipment and injured the experimenter himself.
A vessel with bromine once exploded in the hands of the Russian chemist S.V. Lebedev (1874–1934). Shards of glass and splashes of bromine fell on the hands and face of the scientist, injuring them and accompanying severe burns. Despite the timely assistance provided, some of the fragments remained in Lebedev's body and were surgically removed only three years later.
Speaking about explosions in the laboratory, it is impossible not to mention the German chemist Justus Liebig (1803–1873), who accompanied the explosions throughout almost the entire period of chemistry studies, starting from childhood, and caused many of his troubles in life.
After Justus was kicked out of school for an explosion that took place right in the classroom, his father arranged for Liebig as an apprentice pharmacist. But even here he did not stay long. After a strong explosion that blew off the roof over the attic, in which a 15-year-old boy conducted experiments with explosive mercury (mercury fulminate) ![]() , Justus was expelled and from the pharmacy.
, Justus was expelled and from the pharmacy.
At an older age, Liebig wanted to somehow decompose the explosive silver with ammonium sulfide. However, as soon as the first drop of the solution fell into a cup of explosive silver, a deafening explosion was heard. Liebig was thrown onto his back, lost his hearing for two weeks and nearly went blind. Already a mature scientist, Justus once demonstrated at a lecture the combustion of carbon disulfide vapors in nitric oxide (II). Suddenly, a powerful explosion occurred, fragments of the flask, where the reaction took place, showered all those present. Liebig was lucky again: the largest splinter hit the snuffbox in the scientist's pocket.
Unfortunately, not all chemists were as lucky as Liebig. As a result of poisoning with arsenic, which got into the lungs and the esophagus during the explosion of the retort, the famous mineralogist and chemist, Academician of the Petersburg Academy of Sciences IG Leman (1719–1767) died. Another Russian academician, NP Sokolov (1748-1795), died of poisoning with phosphorus and arsenic while studying the properties of compounds of these elements. Another Russian chemist, a former serf, SP Vlasov (1789–1821) died as a result of poisoning received during chemical research.
During the explosion that occurred during the distillation of coal tar, the English scientist Charles Mansfield (1819-1855) received severe burns from which he died a few days later.
In 1891, at the Main Artillery Range near St. Petersburg, when testing picric acid (2,4,6-trinitrophenol-1)

the explosion killed a full member of the Russian Physicochemical Society, a private teacher of chemistry in the Corps of Pages and at the Pavlovsk Military School, Staff Captain of the Guards Artillery S.V. Panpushko - the author of the first in Russia Collection of Chemistry Problems Explaining Their Solution "and fundamental work" Gunpowder Analysis ".
 |
The life of the talented Russian scientist VE Bogdanovskaya (1867–1896), the author of the Primary Textbook of Chemistry, as well as a number of novellas and short stories, was tragically cut short. During an attempt to obtain a phosphoric analogue of hydrocyanic acid, an ampoule explosion occurred, the glass of which injured Bogdanovskaya's hand. As a result of poisoning with toxic substances, four hours after the explosion, she died.
It was already mentioned above how much trouble the study and work with substances such as mercury or chlorine brought to scientists. However, among the simple substances, fluorine caused the most trouble for researchers. This element turned out to be truly fatal for a number of chemists from different countries. It has already been written about the poisoning with hydrogen fluoride by G. Davy (1778–1829). Trying to isolate fluorine, the French J. Gay-Lussac, L. Tenard, E. Fremy and the Englishman G. Gore seriously undermined their health, the Belgian chemist P. Layet paid with his life, the French scientist D. Nichles was martyred. The attempts to obtain fluorine by isolating it from silver and lead fluorides, undertaken by the English chemists by the Knox brothers, ended tragically: George became disabled, and Thomas died. Other scientists who tried to isolate this element in a free form also suffered to one degree or another.
 |
Only the French scientist A. Moissant (1852-1907) in 1886 managed to accomplish what others were unable to do. However, we note that for him, the solution to this problem did not pass without a trace. When Moissan reported to the Paris Academy of Sciences about his discovery, one of the scientist's eyes was covered with a black bandage.
The accidents listed above happened to famous chemists. And how many explosions and poisonings occurred among lesser-known researchers and novice experimenters! How many injuries, burns and injuries were received!
The study of the phenomenon of radioactivity also brought a lot of trouble to scientists. Radiation, by its very nature, is life-threatening. At high doses, it causes serious tissue damage, leading to rapid death of the body, and at low doses, it can lead to cancer or genetic changes.
One of the first to encounter the effect of radioactive radiation on the tissues of a living organism was the discoverer of the phenomenon of radioactivity, the French scientist A.A. Becquerel (1852-1908). After carrying a test tube with a radium salt in his waistcoat pocket for some time, in April 1901 he received a skin burn. Telling the Curies about this, Becquerel exclaimed: "I love radium, but I am mad at it!"
The life of the English scientist W. Ramzai (1852-1916) was significantly shortened by his work with radium, radon and other radioactive substances. In 1915, the scientist fell ill with lung cancer and died a year after a major operation.
Work with radioactive substances also greatly affected the health of Maria Sklodowska-Curie (1867-1934). First, she underwent severe kidney surgery, then her vision deteriorated sharply, hearing problems appeared. In 1920, in a letter to her sister, she wrote: “My eyesight has become very weak, and this will probably be of little help. As far as hearing is concerned, I am haunted by constant tinnitus, sometimes very loud. " In the period from 1923 to 1930, Maria underwent four operations on her eyes, which eventually restored her vision.
Sklodowska-Curie died on July 4, 1934 from acute malignant anemia caused by degeneration of the bone marrow. In the medical report, Professor Rego wrote: "Madame Curie can be considered one of the victims of long-term handling of radioactive substances that her husband and herself discovered."
They buried Sklodowska-Curie with special precautions. The wooden coffin was placed in a lead one, and that, in turn, in another wooden one. When in 1995 the remains of the outstanding scientist were transferred to the Pantheon, measurements of the radiation level of the inner coffin showed that it was 30 times higher than the background levels.
O the examples written above, although accompanied by very serious consequences, nevertheless mainly concerned only the researchers themselves who conducted the experiments. Unfortunately, there are cases when during chemical experiments the number of victims was much higher. May 27, 1920 became the "black day" in the history of chemistry. On this day, during a demonstration of experiments at high temperatures at the University of Münster (Germany), a violent explosion occurred, as a result of which ten students died and over twenty were injured.
And how many people died as a result of explosions at chemical plants! One of the first such accidents was the explosion at the gunpowder factory in Esson in 1788, during which several people died, and the French chemists Berthollet and Lavoisier, who arrived at the factory, survived only because they decided to inspect the adjacent room at that time. The reason for the explosion was an attempt to replace potassium nitrate in the powder with potassium chlorate.
In 1848 in Le Bourget in France the first plant for the production of pyroxylin - cellulose trinitrate [C 6 H 7 O 2 (ONO 2) 3] n was blown up.
On September 3, 1864, at noon, a terrifying explosion demolished the C 3 H 5 (ONO 2) 3 nitroglycerin factory located near Stockholm and owned by the inventor of dynamite, the Swedish engineer Alfred Nobel. The explosion killed Alfred's younger brother Oscar, as well as the inventor's closest friend, the chemist Hetzman.
In 1887, in England, near Manchester, there was a violent explosion at a dyeing factory using picric acid compounds as a yellow paint.
However, all of the above cases cannot be compared with the explosions that occurred on December 6, 1917 at a chemical plant in Halifax (Canada), September 21, 1921 at a fertilizer plant in Oppau (Germany) and December 2, 1984 d. at a pesticide plant in the Indian city of Bhopal.
In the first case, the explosion, which occurred as a result of the self-decomposition of ammonium nitrate, cost the lives of 3,000 people, in the second, 560 people died and more than 7,500 were left homeless. The explosion in Oppau was so powerful that it not only completely destroyed all the houses in the city itself, but also damaged some buildings 6 km from the explosion site. Moreover, the blast wave knocked out glass in houses located at a distance of 70 km from the plant.
An explosion at a pesticide plant in Bhopal released a large amount of methyl isocyanate CH 3 –N = C = O, a poisonous substance with a pungent odor and high reactivity, into the environment. As a result of the accident, 2,352 people died, 90,000 people were poisoned, and about 150,000 people left the city in panic.
Let us also mention the tragedy that took place in July 1976 in Italy. Dioxin was released into the atmosphere as a result of an accident at a chemical plant in the village of Seveso, near Milan.

This is one of the most potent poisons, the action of which exceeds in its strength hydrocyanic acid, strychnine and curare poison. Hundreds of people were poisoned and hospitalized. Their skin was covered with eczema, ulcers and burns, they were tormented by vomiting, stomach cramps and upset. All vegetation in the vicinity of Seveso, including crops, was burned like a fire, and the land itself became dangerous to people and livestock for decades.
V The overwhelming majority of the accidents listed above, which occurred in laboratories or in chemical industries, tragedies came as a surprise to a researcher or technologist. However, often, not having at hand other organisms besides his own, and eager to quickly study the properties of a new substance, the scientist set up an experiment on himself, sacrificing health, and sometimes life itself for the sake of comprehending the truth. In justifying their actions, such chemists claimed that science required sacrifice, and continued dangerous experiments as long as they could work in the laboratory.
Let us recall again K. Scheele, T. Lovitz, K. Claus, who determined the taste of chemicals. Let us recall G. Davy, D. Woodhouse, U. Kruikshank, who studied the effect of gases on their own body. Let us recall hundreds of other well-known and unknown chemists who were engaged in similar studies. Here are some more examples from this area.
Once a French naturalist of the eighteenth century. Jean François Pilatre de Rozier was interested in the question: what happens if hydrogen is inhaled? Without initially feeling any effect, the scientist decided to make sure that hydrogen penetrated into the lungs. To do this, he once again inhaled the gas, and then exhaled it into the fire of a candle. There was a deafening explosion. “I thought that all my teeth would fly out along with the roots,” the scientist later wrote about the experiment that almost cost him his life.
In an effort to prove the safety of activated carbon for the body, Lovitz conducted the following experiment. He burned 100 g of opium, which is a strong drug, and then ate all the resulting coal during the day. Doubters Lovitz suggested doing a similar experiment with any other plant poison.
Unlike Becquerel, who accidentally received a burn as a result of exposure to radium on his skin, P. Curie (1859-1906) voluntarily exposed his hand to the action of this substance. After being exposed to radiation for 10 hours, his skin first turned red, and then a wound formed, which took more than four months to heal, and a white scar persisted for several years.
 |
Ramsay experienced the effects of injections of radioactive radon. Despite the fact that, according to Ramsay, such injections are effective against cancer, apparently, it was they who caused the early death of the scientist.
The American physicist-chemist G. Yuri (1893-1981) also studied the effect of heavy water on himself. Once he even drank a full glass of heavy water. Fortunately, this risky experiment passed without consequences for him.
As we can see from all of the above, the danger during the experiments and the loss of health as a result of chemical experiments in the past were considered almost mandatory attributes of the work of a chemist and were, as it were, planned in advance. In a concentrated form, this idea is expressed in the words of the great German chemist Liebig, who once, giving instructions to the young Kekule, said: “If you want to become a real chemist, you must sacrifice your health. Nowadays, those who, while studying chemistry, do not destroy their health, will not achieve anything in this science. " It follows from this that Liebig not only did not care about maintaining his health, but also did not think about preserving the health of the people around him. The following example is especially indicative in this regard.
After receiving anhydrous formic acid and making sure on his own skin that the acid causes burns, Liebig began to walk around the laboratory and, in order to demonstrate his discovery, he began to burn the hands of students. Near Liebig himself, a large bubble jumped up from the splashing acid on his cheek, but he did not pay any attention to it. Liebig's colleague, the famous German physiologist and biochemist K. Vogt (1817–1895), received the largest portion of the acid that Liebig put on his hand without a shadow of embarrassment. The result of this rash experiment was a white scar, which remained with Vogt for life.
A lot of water has flown under the bridge since that time. In our time, a look at the problems of maintaining health during chemistry classes in comparison with the eighteenth and nineteenth centuries. has changed dramatically. Few people now come up with the idea of tasting unknown substances or burning their hands with acids. No one has a desire to destroy their health. On the contrary, chemists are trying to create conditions in a modern laboratory that maximize their safety.
But the experience of the chemists of the past did not pass without leaving a trace. Sacrificing themselves for the sake of truth, they used their experience to warn future generations of scientists about the dangers of working with this or that substance. On this basis, measures of protection against toxic, explosive and radioactive substances were improved, laboratory equipment was developed, and safer methods of synthesis and analysis were developed.
Currently, despite the high toxicity and danger of many substances, chemists have proven that working with them can be absolutely harmless. In this they are helped by well-thought-out precautions: powerful traction, protective materials (glasses, gloves, aprons, gas masks, screens), the use of manipulators and other protective equipment. All this in combination allows avoiding the harmful effects of toxic substances on the organisms of chemists and thereby creates conditions for them for a long and fruitful life.
APPLICATION
table
Accidents with research chemists
| Surname of the scientist | Years of life | Country | Reason for defeat (poisoning or explosion) |
|---|---|---|---|
Poisoning |
|||
| T. Paracelsus | 1493–1541 | Germany | Mercury and its compounds |
| I. Glauber | 1604–1670 | Germany | Hydrochloric acid, mercury compounds, antimony |
| R.Boyle | 1627–1691 | England | Phosphorus and its compounds |
| I. Newton | 1643–1727 | England | Mercury and its compounds |
| K. Scheele | 1742–1786 | Sweden | Hydrocyanic acid, chlorine, arsenic and mercury compounds |
| U. Kruikshank | 1745–1810 | England | Carbon monoxide, phosgene, chlorine |
| K. Berthollet | 1748–1822 | France | Chlorine, ammonia, hydrogen sulfide, hydrogen cyanide |
| N. Sokolov | 1748–1795 | Russia | Phosphorus, arsenic |
| T. Lovitz | 1757–1804 | Russia | Mercury, chlorine, strontium compounds |
| D. Woodhouse | 1770–1809 | England | Carbon monoxide |
| L. Tenard | 1777–1857 | France | Sublimate, hydrogen fluoride |
| J. Gay-Lussac | 1778–1850 | France | Hydrogen fluoride |
| G. Davie | 1778–1829 | England | Carbon monoxide, methane, hydrogen fluoride |
| J. Berzelius | 1779–1848 | Sweden | Hydrogen selenide |
| K. Claus | 1796–1864 | Russia | Compounds of osmium, ruthenium |
| R. Bunsen | 1811–1899 | Germany | Arsenic compounds |
| E. Fremy | 1814–1894 | France | Hydrogen fluoride |
| A. Bayer | 1835–1917 | Germany | Methyldichloroarsine |
| N. Zelinsky | 1861–1953 | Russia | 2,2 "-Dichlorodiethyl sulfide |
| E. Fischer | 1852–1919 | Germany | Phenylhydrazine |
| U. Ramzai | 1852–1916 | England | Radium, radon |
| Y. Tafel | 1862–1918 | Germany | Acrolein |
| M. Sklodovskaya-Curie | 1867–1934 | France | Radium, polonium |
Explosions |
|||
| I. Lehman | 1719–1767 | Russia | Arsenic |
| K. Berthollet | 1748–1822 | France | Bertoleth's salt |
| G. Davie | 1778–1829 | England | Alkali metals |
| L. Tenard | 1777–1857 | France | KOH and Fe |
| J. Gay-Lussac | 1778–1850 | France | KOH and Fe |
| P. Dyulong | 1785–1838 | France | Nitrogen (III) chloride |
| Y. Liebig | 1803–1873 | Germany | Volatile mercury, detonating silver |
| R. Bunsen | 1811–1899 | Germany | Arsenic compounds |
| S. Würz | 1817–1884 | France | PCl 3 and Na |
| C. Mansfield | 1819–1855 | England | Volatile fraction of coal tar |
| L. Meyer | 1830–1895 | Germany | Acetylene-air mixture |
| V. Bogdanovskaya | 1867–1896 | Russia | Phosphine |
REFERENCES
Manolov K. Great chemists. T. 1-2. M .: Mir, 1985;
Volkov D.N., Vonsky E.V., Kuznetsova G.I. Outstanding chemists of the world. M .: Higher school, 1991; Stepin B.D., Alikberova L.Yu... Chemistry book for home reading. M .: Chemistry, 1994;
Klyuchevich A.S. Karl Karlovich Klaus. Kazan: Kazan University Publishing House, 1972;
Figurovsky N.A., Ushakova N.N.... Tovy Egorovich Lovits. Moscow: Nauka, 1988;
Mogilevsky B.L. Live in danger! The story of the great chemist Humphrey Davy. M .: Children's literature, 1970;
Curie E. Maria Curie. M .: Atomizdat, 1973;
Krasnogorov V. Justus Liebig. M .: Knowledge, 1980;
D.N. Trifonov, V.D. Trifonov How the chemical elements were discovered. M .: Education, 1980; Soloveichik S. Carelessness costing life. Chemistry and Life, 1966, no. 6, p. 29;
Demidov V.I. Bitter honey - melinitis. Chemistry and Life, 1974, no. 8, p. 61;
Kolchinsky A.G. TB lessons. Chemistry and Life, 1990, no. 2, p. 79;
Zyablov V. Two legends about Tobiya Lovitz. Chemistry and Life, 1977, no. 4, p. 79.
Task 8-1.
Read the text carefully and think about which word, from the proposed list of terms, can replace the spaces in the text indicated by numbers. In this case, words can be changed, put in the desired case and number (for example: substance, substances, substances, etc.). Some words will come in handy several times, others may not be needed even once. Make a list on a draft of what word you will replace each number with. After that, rewrite the text into a clean copy, inserting the necessary words.
Water and oxygen
Water is widespread ... (1). Distilled water is used in laboratories, it is pure ... (2), since all impurities are removed from it. Unlike distilled water, tap water, river or sea water is ... (3), since they contain other substances.
The smallest particle of water is called ... (4), and consists of two ... (5) hydrogen and one ... (6) oxygen. Thus, water consists of two chemical ... (7) - hydrogen and oxygen, therefore it is ... (8) a substance. This is how it differs from the substance necessary for breathing, oxygen. An oxygen molecule consists of two ... (9) oxygen. There are no other chemical ... (10) in the composition of oxygen, therefore oxygen ... (11) is a substance. Oxygen is a part of air, air is ... (12) different gases.
List of terms: substance, body, mixture, compound, atom, molecule, element, complex, pure, simple, dirty.
(12 points)
Task 8-2.
Fish species such as trout and grayling are very sensitive to the purity of the water. If 1 m 3 of river water contains only 0.003 mol of sulfuric acid H 2 SO 4, which can get into the water from "acid rain", then the fry of these fish die. Calculate the mass of sulfuric acid in 1 m 3 of water, which is a lethal dose for the fry of these fish. How many molecules of sulfuric acid will be in one glass of such water (200 cm 3)? Is it more or less the number of centimeters separating Tyumen from Moscow (2200 km)?
(8 points)
Task 8-3.
The teacher prepared samples of different substances for the chemistry lesson. But a playful kitten got to them, as a result everything was mixed in one heap: salt crystals, copper, iron and sawdust. Describe the sequence of steps you can use to separate this mixture and return all substances to separate jars.
What processes, physical or chemical, were used in your proposed method for separating the mixture? What properties of substances, physical or chemical, were used in this case?
(10 points)
Task 8-4.
Two scientists investigated substances obtained in their laboratories. One, using physical methods, found that the molecule of his substance A contains 2 carbon atoms, six hydrogen atoms and one oxygen atom.
Another, using chemical methods, determined that 5 grams of its substance B contains 2.61 g of carbon, 0.652 g of hydrogen and also oxygen. Determining the molecular weight of a substance, he received the same value as the first scientist.
Try to carry out the calculations that these scientists should have done. Is the data obtained sufficient to assert that they studied the same substance?
) conducted a study on the use of high-energy nitrogen-oxygen compounds in organic synthesis. The energy contained in these unstable compounds can be used to build new, more stable chemical bonds. Using this approach, it was possible to obtain biologically active substances containing nitrogen, including drugs. Research supported grant Russian Science Foundation (RSF). The article was recently published in the German magazine Synthesis.
Scientists have investigated the properties of nitronates. In addition to the hydrocarbon chain, these organic compounds contain an unstable chemical group consisting of two oxygen atoms and one nitrogen atom. When heated, such an unstable group disintegrates with the release of a large amount of energy, so these compounds are usually considered high-energy (explosive).
“In our research, we use the high energy contained in unstable nitrogen-oxygen compounds, not for the purpose of destruction, but for creation at the molecular level. Using controlled chemical processes, it is possible to achieve destruction (destruction) of the nitrogen-oxygen fragment in such a way that the released energy is used to build new stable chemical bonds in molecules, ”explains one of the authors of the study, Ph.D., Senior Researcher of the Institute of Organic Chemistry, Russian Academy of Sciences.
Hydrocarbons enter into a small number of reactions, that is, they are chemically relatively inert. In a hydrocarbon chain, it is difficult to replace one of the carbons with another atom (for example, oxygen or nitrogen) or to "assemble" several small molecules into a complex structure. If, however, the molecules are "activated" by the nitro group, thus obtaining the nitronate, these tasks can be accomplished with ease.
Most nitronates are unstable only at elevated temperatures, so it is safe to work with them at room temperatures. The methods used in the study include the use of Lewis acids and transition metal compounds in the reactions. Lewis acids are widely used as catalysts - substances that accelerate chemical reactions many times over. In this study, Lewis acids were used to activate compounds at temperatures no higher than room temperature. The catalysts and experimental conditions varied depending on the specific reaction and the target product.
It is important that due to the use of nitronates as key intermediates, only one optical isomer (or stereoisomer) of the synthesized compound can be obtained. Many complex organic molecules have stereoisomers - molecules that are identical in chemical composition and structure, but differ from each other in the arrangement of groups of atoms. If there is one carbon atom in a molecule, to which four different substituents are attached, such a molecule can have two optical isomers - two forms that are mirror images of each other, like left and right gloves.
Usually, in terms of physical and chemical properties, optical isomers practically do not differ, but biological activity very much depends on which isomer entered the body. For example, we are able to taste the difference between the sweet sugar substitute aspartame and its bitter stereoisomer, although they differ only in which direction the parts of the molecule are directed. Cells perceive all substances that enter the body with the help of receptors. These are large, usually proteinaceous molecules that are located on the outer part of the cell membrane. In order for the cell to react to the presence of a substance, it must bind to receptor proteins, which, in turn, are also asymmetric molecules. The "wrong" optical isomer does not fit the receptor protein for the same reason that the left glove does not fit the right hand. This is very important in the manufacture of medicines.
In conventional chemical synthesis, both forms are most often obtained in equal quantities. To obtain only one optical isomer, it is necessary to use the methods of asymmetric catalysis. And this is where nitrogen-oxygen systems are used. Reactions with nitronates using certain catalysts make it possible to obtain biologically active compounds in a stereo direction, that is, in the form of one optical isomer required by the body.
The use of nitronates has already made it possible to obtain new nitrogen-containing biological substances, as well as to make the process of creating already known compounds more efficient. For example, scientists have synthesized new inhibitors of phosphodiesterase-4. These substances are promising drugs for chronic obstructive pulmonary disease - restriction of airflow in the airways due to inflammation of the lung tissue. The use of nitronates makes it possible to reduce the number of steps in the production of pharmaceutical substances, such as baclofen and phenibut, which are already used as medicines. There is also a search for more effective substitutes for already known biologically active substances.
A group of scientists from the Institute of Organic Chemistry of the Russian Academy of Sciences is working on several problems. Firstly, it is the expansion of the range of transformations and the palette of the resulting products. Scientists are trying to apply those reactions that have already been discovered for the synthesis of already existing practically significant compounds and their analogs. Second, the fundamental features of the behavior of nitronates are investigated, thanks to which new methods of organic synthesis can be created.
“We hope that in the future the methodology that we are developing will take its rightful place in applied organic synthesis,” concludes Alexey Sukhorukov.
Question: Two scientists investigated substances obtained in their laboratories. One, using physical methods, found that the molecule of his substance A contains 2 carbon atoms, six hydrogen atoms and one oxygen atom. Another, using chemical methods, determined that 5 grams of its substance B contains 2.61 g of carbon, 0.652 g of hydrogen and also oxygen. Determining the molecular weight of a substance, he received the same value as the first scientist. In their correspondence, they agreed to calculate and compare the mass fractions of elements in their compounds. Also, the second scientist promised to establish the formula for his substance. Try to carry out the calculations that these scientists should have done. Is the data obtained sufficient to assert that they studied the same substance?
Two scientists investigated substances obtained in their laboratories. One, using physical methods, found that the molecule of his substance A contains 2 carbon atoms, six hydrogen atoms and one oxygen atom. Another, using chemical methods, determined that 5 grams of its substance B contains 2.61 g of carbon, 0.652 g of hydrogen and also oxygen. Determining the molecular weight of a substance, he received the same value as the first scientist. In their correspondence, they agreed to calculate and compare the mass fractions of elements in their compounds. Also, the second scientist promised to establish the formula for his substance. Try to carry out the calculations that these scientists should have done. Is the data obtained sufficient to assert that they studied the same substance?
Answers:
Similar questions
- In the basket Lyuba put 2 bunches of carrots, 7 pieces each, how many carrots are in the basket
- 6th grade, 4 and 5 rooms are needed, thanks in advance)
- An album for drawing is 8 times more expensive than a pencil, and together they cost 135 rubles. How much does an album cost?
- Two rays BD and BK are drawn from the vertex of the unfolded angle ABC so that the angle ABK = 128 ° angle CBD = 164 ° Calculate the value of the angle DBK
- Which of the following is the physical body? drop of water mole steel sunrise. 2K ?? which of the physical bodies can not be connected by compression? 3brutsk plasticine pieces of cast iron shards of glass drops of water. 3
Russian scientists studied particles of meteorite matter and came to the conclusion that microorganisms that came to Earth from space are one and a half billion years older than terrestrial life forms. This means that life on Earth could have originated much later than on other planets.
Every day, from 100 to 1000 tons of extraterrestrial matter falls on our planet from outer space - in the form of dust and meteorites. Experts of the Paleontological Institute of the Russian Academy of Sciences, having examined the structure of space messengers, found in them what, in fact, all mankind has long hoped to find in the Universe - traces of life!
Humanity has always been interested in what is happening outside the Earth, and one of the main questions that haunt us: is there or was there life far from our planet? The question of the existence of extraterrestrial life has been repeatedly raised by scientists from different countries. A new round of research activity in this direction began in 1996, when a group of American scientists led by David McKay published an article in which it was suggested that inside some meteorites, presumably of Martian origin, there are traces of fossil bacteria. It followed from this work that if now there is no life on Mars, then once in distant times it could well have been there at a primitive level.
Since the publication of McKay's publication, researchers have accumulated a huge amount of new material on this topic. For example, by the end of this year, specialists from the Paleontological Institute of the Russian Academy of Sciences, together with colleagues from NASA, are going to publish the Atlas of Biomorphic Structures, which will summarize all the information of recent years. The publication is planned to be composed of two parts. The first will be devoted to organic residues in the rocks of the Earth, and the second - to biomorphic structures in meteorites. Alexey Rozanov, director of the Paleontological Institute of the Russian Academy of Sciences, Doctor of Geological and Mineralogical Sciences, told Itogi about what was still unusual to be seen in the structure of meteorites.
Space parcels
According to their composition, all meteorites that have fallen to the Earth can be roughly divided into stone, iron and iron-stone. Scientists find the remains of biomorphic structures only in one of the varieties of stone meteorites - carbonaceous chondrites (they got this name from the chondrules - spherical silicate formations in their structure). Solving the problem of the origin of carbonaceous material in such meteorites is fundamentally important, since the development of ideas about the origin of life in general and on Earth in particular depends on it. And therefore it is not surprising that the main objects for scientific work were precisely stone meteorites of a similar type - Efremovka (found in Kazakhstan in 1962) and Murchison (Australia, 1969). Using an electronic microanalyzer, specialists examined the composition of the mineral matrix, first of one, and then of the second meteorite. And they found the following: in both cases, inside the matrix there were fossil particles of filamentous microorganisms that had preserved the details of the cellular structure, resembling lower fungi, as well as (and this is most important!) Fossilized remains of certain bacteria.
It was possible to compare biomorphic structures found in meteorites with modern microorganisms, as well as with samples of the bacterial world of antiquity. These experiments laid the foundation for a new direction in science - "bacterial paleontology". As the paleontologists themselves say, this is another key to deciphering cosmic organic material. Modern terrestrial analogs of microorganisms found in meteorites turned out to be blue-green algae, or cyanobacteria.
For reference: cyanobacteria are the oldest photosynthetic organisms, the vital activity of which, as is known for certain to science, unloaded the ancient atmosphere of the Earth from carbon dioxide and provided it with oxygen. It was cyanobacteria, together with their accompanying bacteria, that for more than three billion years became the complete masters of the Earth and largely determined the course of such important geological events as the accumulation of many sedimentary rocks and minerals. The communities created by these microorganisms, which have close metabolic links, have proven remarkably stable throughout the entire history of the Earth. True, more highly organized competitors gradually ousted them from wide sea spaces into ecological niches, mainly with extreme conditions, such as hypersaline lagoons, volcanic areas. And in these places, microbial communities are preserved to this day.
Thus, the presence of analogs of cyanobacteria in the carbonaceous matter of meteorites forced the scientific community to recognize the undoubted fact of their biogenic origin. What does this prove? The fact that the significant morphological unity of terrestrial microbial organisms, both modern and ancient, with formations in meteorites gives grounds to speak of the fundamental unity of the microbiological world of the Earth and other space objects.
The remains of microorganisms, probably belonging to cyanobacteria, may also indicate the sensational fact that the formation of the substance of carbonaceous chondrites took place in an aquatic environment. This inevitably leads to the conclusion that at least 4.5-4.6 billion years ago, somewhere outside the Earth, life existed at least at the level of bacteria and, perhaps, lower fungi. This age is comparable to the time of the beginning of the formation of the Earth. On this basis, paleontologists concluded that somewhere in space the bacterial world appeared earlier than on our planet. And who would undertake to deny that he could develop further along a completely different, unearthly path? Perhaps, somewhere on distant planets, such life forms have formed that are fundamentally different from terrestrial ones and about which modern science has not the slightest idea. Someone will call it a fantasy, but how can you not remember that until recently the possibility of the presence of water on Mars was considered absurd.
“The discovery of microorganisms in stony meteorites forces us to significantly reconsider many of the established ideas about the development of the solar system and the origin of life,” says Alexey Rozanov. “And one more important point: the age of microorganisms gives us the opportunity to fight the misconception that space bodies are carriers of dangerous bacteria. Fossilized microbes that come to Earth in meteorites are harmless because they have been dead for several billion years. "
The next stage of fascinating research was associated with the study of the process of fossilization of microorganisms. And here scientists also expected unexpected results. “The results of laboratory experiments were stunning, - says Alexey Rozanov. - It turned out that the fossilization process can take only a few hours. Previously, we assumed that all fossil organisms were petrified for almost millions of years. But it turned out that this was not at all a mandatory requirement. the speed of this process explains why the bacteria we find in ancient stones are so well preserved. "
Another proof that bacteria, and not something else, are present in the meteorites that have fallen to the Earth, was the discovery in them of magnetite crystals and spherical bodies consisting of small crystals (framboids). The fact is that on Earth such bizarre structures are formed only with the direct participation of microorganisms.
Despite the fact that the research of paleontologists in this direction is progressing rather quickly, certain difficulties nevertheless arise on their way. So, for example, opinions are expressed that it is hardly possible to speak about the purity of experiments, since meteorites can be "clogged" by terrestrial microorganisms. Specialists of the Paleontological Institute agree that, upon entering our planet, space bodies are exposed to the penetration of microorganisms into them, but they do not consider this problem insoluble. Knowing approximately the composition of the meteorite substance, scientists have learned to determine to what extent terrestrial microorganisms have mastered space artifacts. If the amount of any component in a meteorite goes beyond its possible content, then it is hopelessly "clogged".
“During our research, we analyzed almost two dozen meteorites, and in almost all cases ancient fossils were found,” says Alexey Rozanov. “Without a doubt, microorganisms are similar to those bacteria that live today and those that are in the fossil On the basis of research data, we can confidently assert that the microorganisms in the composition of meteorites are ancient bacteria. we do not exclude the possibility of detecting in the future such forms that will not have terrestrial analogues. "
Hard to believe
The conclusions of Alexei Rozanov are very unusual and therefore are not unambiguously accepted in the scientific community. "Itogi" were able to be convinced of this, having talked with the main opponents of the respected scientist. So, for example, the head of the meteoritics laboratory of the Institute of Geochemistry and Analytical Chemistry. VI Vernadsky RAS, Doctor of Geological and Mineralogical Sciences Mikhail Nazarov firmly stands on the fact that today there are no reliable facts that would indicate the possibility of the presence of organic matter residues in meteorites: “This issue has been repeatedly studied, and there are people who believe For example, Alexei Yuryevich Rozanov. He believes that he has found some remnants of microorganisms. But I do not think that this thing is one hundred percent proven. "
And here is the opinion of Alexander Ulyanov, Doctor of Geological and Mineralogical Sciences, Professor of the Department of Mineralogy at Moscow State University, member of the Committee on Meteorites of the Russian Academy of Sciences: “I am familiar with Rozanov's point of view. Yuryevich studied carbonaceous chondrite Efremovka, in which he allegedly found organic matter - something resembling fossilized bacteria, but at the same time this meteorite was lying in the fields, which were fertilized with various active components for probably forty years. oxidation with iron is noticeable. Therefore, I do not consider this finding as reliable. But this is exclusively my point of view. Moreover, I do not believe in the detection of microorganisms inside Martian meteorites and I consider such statements unreliable and unsubstantiated. "
Did ancient bacteria come from space or did they originate on Earth? We will receive the answer to this question only after scientific research comes to its end. However, it is already clear today that new ways of searching for life in the Universe are forcing science to revise the established ideas about the development and origin of the solar system.
Ekaterina Gorbunova
BACKGROUND
Controversial science
On March 15, 1806, a stone meteorite fell in the town of Alais (France). It was the first carbonaceous chondrite to be extensively studied. So, in 1834, the Swedish chemist Berzelius, having studied his sample, was surprised when he found water in it, and also noted the similarity of the meteorite's carbonaceous matter with terrestrial biological material.
On May 14, 1864, more than 20 black stones (some weighing about 2 kg) fell near the French villages of Noic and Orgei. In the immediate aftermath of the fall, the villagers collected bluish-black stones, many of which were completely crusty. The Orgei meteorite was immediately subjected to a thorough chemical and mineralogical analysis. The carbon content in its fragments was so high that at first this fact was considered as a consequence of pollution by terrestrial matter. However, later it was concluded that the participation of living material in the formation of the meteorite is very likely.
The hypothesis of the existence of extraterrestrial "life-like" forms in meteorites, first put forward in the middle of the 19th century, was widely accepted and successfully existed for almost a century - until the 60s of the 20th century. In 1962, American researchers Anders and Fitch opposed the biogenic nature of the meteorite material, stating that the fossils in them had no analogues and therefore the biogenic nature should be rejected. They assumed that the imaginary microorganisms were not biological objects, and considered all other biologically similar bodies to be terrestrial pollution - "museum dust" and "pollen". Anders and Fitch are still considered the most active critics of the version about the presence of microorganisms in meteorites.
In 1964, Soviet scientist Boris Timofeev published an article in Germany on the discovery in the Migei meteorite of formations resembling terrestrial phytoplankton. The article was smashed to smithereens. By the way, among the critics was Alexei Rozanov, who today, according to him, has changed his point of view on this publication.
In 1966, Nobel Prize winner in chemistry GK Urey reviewed the evidence for biological materials in meteorites. He noted that organic substances are found in meteorites that closely resemble those of ancient earth rocks, that the organic matter found in carbonaceous chondrites does not resemble that found in modern pollution. Yuri noted: "... some substances in meteorites, if found in terrestrial objects, would undoubtedly be considered biogenic."