Electric current in gases
Charge carriers: electrons, positive ions, negative ions.
Charge carriers arise in a gas as a result of ionization: due to irradiation of the gas, or collisions of particles of a heated gas with each other.
Electron impact ionization.
A_ (fields) = eEl
e = 1.6 \ cdot 10 ^ (19) Cl;
E is the direction of the field;
l is the mean free path between two successive collisions of an electron with gas atoms.
A_ (fields) = eEl \ geq W - ionization condition
W is the ionization energy, i.e. energy required to rip an electron out of an atom

The number of electrons increases exponentially, resulting in an electron avalanche, and hence a discharge in the gas.
Electric current in a liquid
Liquids, just like solids, can be dielectrics, conductors and semiconductors. Distilled water is among the dielectrics, and solutions of electrolytes: acids, alkalis, salts and metal melts are conductors. Liquid semiconductors are molten selenium and sulphide melts.
Electrolytic dissociation
When electrolytes dissolve under the influence of the electric field of polar water molecules, electrolyte molecules decompose into ions. For instance, CuSO_ (4) \ rightarrow Cu ^ (2 +) + SO ^ (2 -) _ (4).
Along with dissociation, the opposite process is going on - recombination , i.e. combining ions of opposite signs into neutral molecules.
The carriers of electricity in electrolyte solutions are ions. This conductivity is called ionic .
Electrolysis
If electrodes are placed in a bath with an electrolyte solution and a current is applied, then negative ions will move to the positive electrode, and positive ions to the negative one.
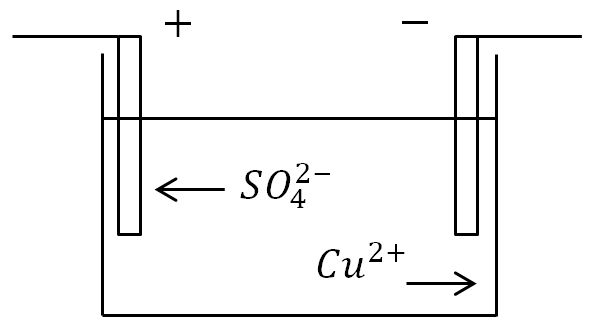
At the anode (positive electrode), negatively charged ions donate excess electrons (oxidative reaction), and at the cathode (negative electrode), positive ions receive missing electrons (reduction reaction).
Definition. The process of release of substances on the electrodes associated with redox reactions is called electrolysis.
Faraday's laws
I. The mass of the substance that is released at the electrode is directly proportional to the charge flowing through the electrolyte:
m = kq
k is the electrochemical equivalent of the substance.
q = I \ Delta t, then
m = kI \ Delta t
k = \ frac (1) (F) \ frac (\ mu) (n)
\ frac (\ mu) (n) - chemical equivalent of a substance;
\ mu - molar mass;
n - valence
Electrochemical equivalents of substances are proportional to chemical ones.
F is the Faraday constant;
The origin of electric current (movement of electric charges) through a solution is significantly different from the movement of electric charges along a metal conductor.
The difference, first of all, is that the charge carriers in solutions are not electrons, but ions, i.e. the atoms or molecules themselves that have lost or captured one or more electrons.
Naturally, this movement, one way or another, is accompanied by a change in the properties of the substance itself.
Consider an electrical circuit, an element of which is a vessel with a solution of sodium chloride and with electrodes of any shape from a plate inserted into it. When connected to a power source, a current appears in the circuit, which is the movement of heavy charged particles - ions in the solution. The appearance of ions already means the possibility of chemical decomposition of the solution into two main elements - Na and Cl. Sodium that has lost an electron is a positively charged ion moving towards an electrode that is connected to the negative pole of a power source, an electrical circuit. Chlorine, which "usurped" an electron, is a negative ion.
Negative chlorine ions move to the electrode, which is connected to the positive pole of the power source. chains.
The formation of positive and negative ions occurs due to the spontaneous decomposition of the salt molecule in an aqueous solution (electrolytic dissociation). The movement of ions is caused by the voltage applied to the electrodes dipped into the solution. Having reached the electrodes, the ions take or donate electrons, forming the Cl and Na molecules, respectively. Similar phenomena are observed in solutions of many other substances. The molecules of these substances, like the molecules of table salt, consist of oppositely charged ions, into which they decompose in solutions. The number of disintegrated molecules, more precisely, the number of ions, characterizes the electrical resistance of the solution.
We emphasize once again that the origin of an electric current along a circuit, the element of which is a solution, causes a movement of the substance of this element of an electric circuit, and, therefore, a change in its chemical properties, while during the passage of an electric current through a metal conductor, no changes in the conductor going on.
What determines the amount of substance released during electrolysis on the electrodes? Faraday was the first to answer this question. Faraday showed experimentally that the mass of the released substance is related to the strength of the current and the time of its flow t by the ratio (Faraday's law):
The mass of the released substance during the electrolysis of a substance is directly proportional to the amount of electricity passed through the electrolyte and does not depend on other reasons, except for the kind of substance.
This pattern can be verified in the following experiments. Pour the same electrolyte into several baths, but with different concentrations. Let us put electrodes of different areas into the bathtubs, and place them in the bathtubs at different distances. We connect all the baths in series and let the current flow through them. Then, obviously, the same amount of electricity will pass through each of the baths. Having weighed the cathodes before and after the experiment, we find that the same amount of substance was released on all the cathodes. By connecting all the baths in parallel and passing a current through them, you can make sure that the amount of substance released at the cathodes is directly proportional to the amount of electricity that passed through each of them. Finally, by connecting sequentially baths with different electrolytes, it is easy to establish that the amount of the released substance depends on the kind of this substance.
The value characterizing the dependence of the amount of a substance released during electrolysis on its kind is called the electrochemical equivalent and is denoted by the letter k.
The mass of the substance released during electrolysis is the total mass of all ions discharged at the electrode. By subjecting different salts to electrolysis, one can experimentally establish the amount of electricity that must pass through the electrolyte in order to release one kilogram - the equivalent of a given substance. Such experiments were first performed by Faraday. He found that the release of one kilogram - the equivalent of any substance during electrolysis requires the same amount of electricity, equal to 9.65 107 K.
The amount of electricity required to release a kilogram of a substance equivalent during electrolysis is called the Faraday number and is denoted by the letter F:
F = 9.65 107 k.
In an electrolyte, an ion is surrounded by solvent (water) molecules with significant dipole moments. Interacting with an ion, dipole molecules turn towards it with their ends, which have a charge opposite to the charge of the ion; therefore, the orderly movement of the ion in an electric field is hampered, and the mobility of ions is significantly inferior to the mobility of conduction electrons in the metal. Since the concentration of ions is usually not high in comparison with the concentration of electrons in a metal, the electrical conductivity of electrolytes is always significantly less than the electrical conductivity of metals.
Due to strong heating by current in electrolytes, only insignificant current densities are achievable, i.e. low electric field strength. With an increase in the electrolyte temperature, the ordered orientation of the solvent dipoles deteriorates under the influence of the increased random movement of molecules, therefore, the dipole shell is partially destroyed, the mobility of ions and the conductivity of the solution increase. The dependence of specific electrical conductivity on concentration at a constant temperature is complex. If dissolution is possible in any proportions, then at a certain concentration the electrical conductivity has a maximum. The reason for this is this: the probability of the decomposition of molecules into ions is proportional to the number of solvent molecules and the number of molecules of the soluble substance per unit volume. But the opposite process is also possible: (recombination of ions into molecules), the probability of which is proportional to the square of the number of ion pairs. Finally, electrical conductivity is proportional to the number of ion pairs per unit volume. Therefore, at low concentrations, dissociation is complete, but the total number of ions is small. At very high concentrations, dissociation is weak and the number of ions is also small. If the solubility of a substance is limited, then usually the maximum electrical conductivity is not observed. Upon freezing, the viscosity of an aqueous solution increases sharply, the mobility of ions decreases sharply, and the specific electrical conductivity drops a thousand times. When liquid metals solidify, the electron mobility and electrical conductivity hardly change.
Electrolysis is widely used in various electrochemical industries. The most important of them: electrolytic production of metals from aqueous solutions of their salts and from their molten salts; electrolysis of chloride salts; electrolytic oxidation and reduction; obtaining hydrogen by electrolysis; electroplating; electrotype; electropolishing. By refining, pure metal is obtained, purified from impurities. Electroplating - covering metal objects with another layer of metal. Electroplating - obtaining metal copies from relief images of any surfaces. Electropolishing - leveling metal surfaces.
« Physics - Grade 10 "
What are the carriers of electric current in a vacuum?
What is the nature of their movement?
Liquids, like solids, can be dielectrics, conductors, and semiconductors. Dielectrics include distilled water, conductors - solutions and melts of electrolytes: acids, alkalis and salts. Liquid semiconductors are molten selenium, sulphide melts, etc.
Electrolytic dissociation.
When electrolytes dissolve under the influence of the electric field of polar water molecules, electrolyte molecules decompose into ions.
The disintegration of molecules into ions under the influence of the electric field of polar water molecules is called electrolytic dissociation.
Dissociation degree- the proportion in the dissolved substance of molecules that have decayed into ions.
The degree of dissociation depends on the temperature, the concentration of the solution, and the electrical properties of the solvent.
With increasing temperature, the degree of dissociation increases and, consequently, the concentration of positively and negatively charged ions increases.
Ions of different signs, when they meet, can again combine into neutral molecules.
Under unchanged conditions, a dynamic equilibrium is established in the solution, at which the number of molecules that decay into ions per second is equal to the number of pairs of ions, which again combine into neutral molecules during the same time.
Ionic conductivity.
Charge carriers in aqueous solutions or molten electrolytes are positively and negatively charged ions.
If a vessel with an electrolyte solution is included in an electrical circuit, then negative ions will begin to move to the positive electrode - the anode, and positive ions - to the negative - to the cathode. As a result, an electric current will flow through the circuit.
The conductivity of aqueous solutions or electrolyte melts, which is carried out by ions, is called ionic conduction.
Electrolysis. With ionic conduction, the passage of current is associated with the transfer of matter. On the electrodes, the release of substances that make up the electrolytes occurs. At the anode, negatively charged ions give up their extra electrons (in chemistry, this is called an oxidative reaction), and at the cathode, positive ions receive the missing electrons (reduction reaction).
Liquids can also be electronically conductive. Liquid metals, for example, have such conductivity.
The process of release at the electrode of a substance associated with redox reactions is called electrolysis.
What determines the mass of a substance released over a certain time? Obviously, the mass m of the released substance is equal to the product of the mass m 0i of one ion by the number N i of ions that reached the electrode during the time Δt:
m = m 0i N i. (16.3)
The mass of the ion m 0i is equal to:
where M is the molar (or atomic) mass of the substance, and N A is Avogadro's constant, that is, the number of ions in one mole.
The number of ions reaching the electrode is
where Δq = IΔt is the charge that has passed through the electrolyte during the time Δt; q 0i is the charge of the ion, which is determined by the valence n of the atom: q 0i = ne (e is the elementary charge). During the dissociation of molecules, for example KBr, consisting of monovalent atoms (n = 1), ions K + and Br - appear. Dissociation of copper sulfate molecules leads to the appearance of doubly charged ions Cu 2+ and SO 2-4 (n = 2). Substituting expressions (16.4) and (16.5) into formula (16.3) and taking into account that Δq = IΔt, a q 0i = ne, we obtain
Faraday's law.
Let us denote by k the coefficient of proportionality between the mass m of the substance and the charge Δq = IΔt passed through the electrolyte:
where F = eN A = 9.65 10 4 C / mol - Faraday constant.
The coefficient k depends on the nature of the substance (values of M and n). According to formula (16.6), we have
m = kIΔt. (16.8)
Faraday's law of electrolysis:
The mass of the substance released at the electrode during the time Δt. with the passage of an electric current, it is proportional to the strength of the current and time.
This statement, obtained theoretically, was first established experimentally by Faraday.
The quantity k in formula (16.8) is called electrochemical equivalent of this substance and is expressed in kilograms per pendant(kg / Cl).
From the formula (16.8) it can be seen that the coefficient k is numerically equal to the mass of the substance released on the electrodes during the transfer of a charge equal to 1 C by ions.

The electrochemical equivalent has a simple physical meaning. Since M / N A = m 0i and en = q 0i, then according to formula (16.7) k = rn 0i / q 0i, that is, k is the ratio of the mass of the ion to its charge.
By measuring the values of m and Δq, it is possible to determine the electrochemical equivalents of various substances.
You can be convinced of the validity of Faraday's law by experience. Let's assemble the installation shown in Figure (16.25). All three electrolytic baths are filled with the same electrolyte solution, but the currents passing through them are different. Let us denote the strength of the currents through I1, I2, I3. Then I 1 = I 2 + I 3. By measuring the masses m 1, m 2, m 3 of substances released on the electrodes in different baths, one can make sure that they are proportional to the corresponding strengths of the currents I 1, I 2, I 3.
Determination of the electron charge.
Formula (16.6) for the mass of the substance released on the electrode can be used to determine the electron charge. From this formula it follows that the modulus of the electron charge is equal to:
Knowing the mass m of the released substance during the passage of the charge IΔt, the molar mass M, the valence of n atoms and Avogadro's constant N A, we can find the value of the modulus of the electron charge. It turns out to be equal to e = 1.6 10 -19 C.
It was in this way that the value of the elementary electric charge was obtained for the first time in 1874.
Electrolysis application. Electrolysis is widely used in technology for various purposes. Electrolytically cover the surface of one metal with a thin layer of another ( nickel plating, chrome plating, gold plating etc.). This durable coating protects the surface from corrosion. If you ensure good peeling of the electrolytic coating from the surface on which the metal is deposited (this is achieved, for example, by applying graphite to the surface), then you can get a copy from the embossed surface.
The process of obtaining peelable coatings - electrotype- was developed by the Russian scientist B.S. Jacobi (1801-1874), who in 1836 applied this method to make hollow figures for St. Isaac's Cathedral in St. Petersburg.
Previously, in the printing industry, copies from a relief surface (stereotypes) were obtained from matrices (an imprint of a set on a plastic material), for which a thick layer of iron or another substance was deposited on the matrix. This made it possible to reproduce the set in the required number of copies.
With the help of electrolysis, metals are purified from impurities. Thus, crude copper obtained from the ore is cast in the form of thick sheets, which are then placed in a bath as anodes. During electrolysis, the copper of the anode dissolves, impurities containing valuable and rare metals fall to the bottom, and pure copper settles on the cathode.
With the help of electrolysis, aluminum is obtained from bauxite melt. It was this method of producing aluminum that made it cheap and, along with iron, the most widespread in technology and everyday life.
With the help of electrolysis, electronic boards are obtained, which serve as the basis for all electronic products. A thin copper plate is glued onto the dielectric, on which a complex pattern of connecting wires is applied with a special paint. Then the plate is placed in an electrolyte, where areas of the copper layer not covered with paint are etched out. After that, the paint is washed off, and the details of the microcircuit appear on the board.
Liquids, like solids, can be conductors, semiconductors, and dielectrics. This lesson focuses on conductive fluids. And not about liquids with electronic conductivity (molten metals), but about liquids-conductors of the second kind (solutions and melts of salts, acids, bases). The conductivity type of such conductors is ionic.
Definition... Conductors of the second kind are such conductors in which chemical processes occur when current flows.
For a better understanding of the process of current conduction in liquids, one can imagine the following experiment: Two electrodes were placed in a bath of water, connected to a current source; in the circuit, you can take a light bulb as a current indicator. If you close such a circuit, the lamp will not burn, which means there is no current, which means that there is a break in the circuit, and the water itself does not conduct current. But if you put a certain amount of table salt in the bathroom and repeat the closure, the light will turn on. This means that free charge carriers, in this case, ions, began to move in the bath between the cathode and the anode (Fig. 1).

Rice. 1. Scheme of the experiment
Electrolyte conductivity
Where do free charges come from in the second case? As mentioned in one of the previous lessons, some dielectrics are polar. Water has just the same polar molecules (Fig. 2).

Rice. 2. The polarity of the water molecule
When salt is added to water, water molecules are oriented in such a way that their negative poles are near sodium, and positive ones are near chlorine. As a result of interactions between charges, water molecules break salt molecules into pairs of unlike ions. The sodium ion has a positive charge, the chlorine ion is negative (Fig. 3). It is these ions that will move between the electrodes under the action of an electric field.

Rice. 3. Scheme of the formation of free ions
When sodium ions approach the cathode, it receives its missing electrons, while chlorine ions, when they reach the anode, give up theirs.
Electrolysis
Since the flow of current in liquids is associated with the transfer of matter, with such a current the process of electrolysis takes place.
Definition. Electrolysis is a process associated with redox reactions, in which a substance is released on the electrodes.
Substances that, as a result of such cleavages, provide ionic conductivity are called electrolytes. This name was proposed by the English physicist Michael Faraday (Fig. 4).
Electrolysis makes it possible to obtain substances from solutions in a sufficiently pure form, therefore it is used to obtain rare materials such as sodium, calcium ... in pure form. This is done by the so-called electrolytic metallurgy.
Faraday's laws
In his first work on electrolysis in 1833, Faraday presented his two laws of electrolysis. The first dealt with the mass of the substance released on the electrodes:
Faraday's first law states that this mass is proportional to the charge passed through the electrolyte:
Here the role of the proportionality coefficient is played by the quantity - the electrochemical equivalent. This is a tabular value that is unique for each electrolyte and is its main characteristic. Dimension of the electrochemical equivalent:
![]()
The physical meaning of the electrochemical equivalent is the mass released on the electrode when the amount of electricity in 1 C passes through the electrolyte.
If you recall the formulas from the topic about direct current:
Then you can represent the first Faraday's law in the form:
Faraday's second law directly concerns the measurement of the electrochemical equivalent through other constants for a given electrolyte:
Here: - molar mass of the electrolyte; - elementary charge; - valence of the electrolyte; is Avogadro's number.
The quantity is called the chemical equivalent of the electrolyte. That is, in order to know the electrochemical equivalent, it is enough to know the chemical equivalent, the rest of the formulas are world constants.
Based on the second Faraday's law, the first law can be represented as:
![]()
Faraday proposed a terminology for these ions in terms of the electrode to which they move. Positive ions are called cations because they move towards the negatively charged cathode, negative charges are called anions as they move towards the anode.
The above-described action of water to break a molecule into two ions is called electrolytic dissociation.
In addition to solutions, melts can also be conductors of the second kind. In this case, the presence of free ions is achieved by the fact that at high temperatures very active molecular movements and vibrations begin, as a result of which the destruction of molecules into ions occurs.
Practical application of electrolysis
The first practical application of electrolysis occurred in 1838 by the Russian scientist Jacobi. With the help of electrolysis, he obtained an imprint of figures for St. Isaac's Cathedral. This application of electrolysis is called electroforming. Another area of application is electroplating - coating of one metal with another (chrome plating, nickel plating, gold plating, etc., Fig. 5)
- Fatyf.narod.ru ().
- Chemistry ().
- Ens.tpu.ru ().
Homework
- What are electrolytes?
- What are the two fundamentally different types of liquids in which an electric current can flow?
- What are the mechanisms for the formation of free charge carriers?
- * Why is the mass released on the electrode proportional to the charge?





