The rates of chemical reactions can increase dramatically in the presence of various substances that are not reactants and are not part of the reaction products. This remarkable phenomenon is called catalysis(from the Greek "katalysis" - destruction). A substance that increases the rate of a reaction in a mixture is called catalyst. Its amount before and after the reaction remains unchanged. Catalysts do not represent any special class of substances. In various reactions, metals, oxides, acids, salts, and complex compounds can exhibit a catalytic effect. Chemical reactions in living cells proceed under the control of catalytic proteins called enzymes. Catalysis should be considered as a true chemical factor in increasing the rates of chemical reactions, since the catalyst is directly involved in the reaction. Catalysis is often more powerful and less risky in speeding up a reaction than raising the temperature. This is clearly manifested in the example of chemical reactions in living organisms. Reactions, such as the hydrolysis of proteins, which in laboratories have to be carried out with prolonged heating to the boiling point, during digestion proceed without heating at body temperature.
For the first time, the phenomenon of catalysis was observed by the French chemist L. J. Tenard (1777-1857) in 1818. He discovered that oxides of certain metals, when hydrogen peroxide is added to a solution, cause its decomposition. Such an experience is easy to reproduce by adding crystals of potassium permanganate to a 3% hydrogen peroxide solution. Salt KMp0 4 turns into Mn0 2, and oxygen is quickly released from the solution under the action of oxide:
The direct effect of the catalyst on the reaction rate is associated with a decrease in the activation energy. At normal temperature decrease? and by 20 kJ/mol increases the rate constant by approximately 3000 times. downgrade E L may be much stronger. However, the decrease in the activation energy is an external manifestation of the action of the catalyst. The reaction is characterized by a certain value E. v which can only change if the reaction itself changes. Giving the same products, the reaction with the participation of the added substance proceeds along a different path, through different stages and with a different activation energy. If on this new path the activation energy is lower and the reaction is correspondingly faster, then we say that this substance is a catalyst.
The catalyst interacts with one of the reactants, forming some intermediate compound. At one of the subsequent stages of the reaction, the catalyst is regenerated - it leaves the reaction in its original form. Reagents, participating in a catalytic reaction, continue to interact with each other and along a slow path without the participation of a catalyst. Therefore, catalytic reactions belong to a variety of complex reactions called series-parallel. On fig. 11.8 shows the dependence of the rate constant on the concentration of the catalyst. The dependence graph does not pass through zero, since in the absence of a catalyst, the reaction does not stop.

Rice. 11.8.
observable constant k expressed as a sum k u+ & k c(k)
Example 11.5. At a temperature of -500 °C, the oxidation reaction of sulfur oxide (IV)
which is one of the stages of industrial production of sulfuric acid, is very slow. A further increase in temperature is unacceptable, since the equilibrium shifts to the left (exothermic reaction) and the product yield drops too much. But this reaction is accelerated by various catalysts, one of which may be nitric oxide (II). First, the catalyst reacts with oxygen: ![]()
and then transfers an oxygen atom to sulfur oxide (IV):
Thus, the final product of the reaction is formed and the catalyst is regenerated. For the reaction, the possibility of flowing along a new path was opened, in which the rate constants increased significantly:

The diagram below shows both pathways of the S0 2 oxidation process. In the absence of a catalyst, the reaction proceeds only along the slow path, and in the presence of a catalyst, along both.
There are two types of catalysis - homogeneous and heterogeneous. In the first case, the catalyst and reagents form a homogeneous system in the form of a gas mixture or solution. An example of sulfur oxide oxidation is homogeneous catalysis. The rate of a homogeneous catalytic reaction depends on both the concentrations of the reactants and the concentration of the catalyst.
In heterogeneous catalysis, the catalyst is a solid in pure form or supported on carrier. For example, platinum as a catalyst can be fixed on asbestos, alumina, etc. Reagent molecules are adsorbed (absorbed) from a gas or solution at specific points on the catalyst surface - active centers and are activated at the same time. After the chemical transformation, the resulting product molecules are desorbed from the catalyst surface. Acts of particle transformation are repeated at active centers. Among other factors, the rate of a heterogeneous catalytic reaction depends on the surface area of the catalytic material.
Heterogeneous catalysis is especially widely used in industry. This is due to the ease of carrying out a continuous catalytic process with the passage of a mixture of reagents through a contact apparatus with a catalyst.
Catalysts act selectively, accelerating a very specific type of reaction or even a single reaction without affecting others. This makes it possible to use catalysts not only to speed up reactions, but also to purposefully convert starting materials into desired products. Methane and water at 450 ° C on the Fe 2 0 3 catalyst are converted into carbon dioxide and hydrogen:
The same substances at 850 °C react on the nickel surface to form carbon monoxide (II) and hydrogen:

Catalysis belongs to those areas of chemistry in which it is not yet possible to make accurate theoretical predictions. All industrial catalysts for the processing of petroleum products, natural gas, ammonia production and many others have been developed on the basis of laborious and lengthy experimental studies.
The ability to control the speed of chemical processes is of inestimable importance in human economic activity. In the industrial production of chemical products, it is usually necessary to increase the rates of technological chemical processes, and in the storage of products, it is required to reduce the rate of decomposition or exposure to oxygen, water, etc. Known substances that can slow down chemical reactions. They're called inhibitors, or negative catalysts. Inhibitors are fundamentally different from real catalysts in that they react with active species (free radicals) that, for one reason or another, arise in a substance or its environment and cause valuable decomposition and oxidation reactions. Inhibitors are gradually consumed, ending their protective action. The most important type of inhibitors are antioxidants, which protect various materials from the effects of oxygen.
It should also be reminded of what cannot be achieved with the help of catalysts. They are capable of accelerating only spontaneous reactions. If the reaction does not proceed spontaneously, then the catalyst will not be able to accelerate it. For example, no catalyst can cause water to decompose into hydrogen and oxygen. This process can be carried out only by electrolysis, while spending electrical work.
Catalysts can also activate unwanted processes. In recent decades, there has been a gradual destruction of the ozone layer of the atmosphere at an altitude of 20-25 km. It is assumed that some substances are involved in the decay of ozone, for example, halogenated hydrocarbons emitted into the atmosphere by industrial enterprises, as well as used for domestic purposes.
Catamlis- selective acceleration of one of the possible thermodynamically allowed directions of a chemical reaction under the action of a catalyst (s), which repeatedly enters into an intermediate chemical interaction with the reaction participants and restores its chemical composition after each cycle of intermediate chemical interactions. The term "catalysis" was introduced in 1835 by the Swedish scientist Jöns Jakob Berzelius.
The phenomenon of catalysis is widespread in nature (most of the processes occurring in living organisms are catalytic) and is widely used in technology (in oil refining and petrochemistry, in the production of sulfuric acid, ammonia, nitric acid, etc.). Most of all industrial reactions are catalytic.
Catalysts Substances that change the rate of chemical reactions are called.
Some catalysts greatly accelerate the reaction - positive catalysis, or just catalysis, others slow down - negative catalysis. Examples of positive catalysis are the production of sulfuric acid, the oxidation of ammonia to nitric acid using a platinum catalyst, etc.
According to the influence on the reaction rate, catalysis is divided into positive (the reaction rate increases) and negative (the reaction rate decreases). In the latter case, an inhibition process takes place, which cannot be considered "negative catalysis", since the inhibitor is consumed during the reaction.
Catalysis can be homogeneous and heterogeneous (contact). In homogeneous catalysis, the catalyst is in the same phase as the reactants, while heterogeneous catalysts differ in phase.
homogeneous catalysis.
An example homogeneous catalysis is the decomposition of hydrogen peroxide in the presence of iodine ions. The reaction proceeds in two stages:
H2 O2+I> H2O+IO, H2O2+io> H2O + O2+ I
In homogeneous catalysis, the action of the catalyst is due to the fact that it interacts with the reactants to form intermediate compounds, which leads to a decrease in the activation energy.
heterogeneous catalysis.
In heterogeneous catalysis, the acceleration of the process usually occurs on the surface of a solid body - the catalyst, so the activity of the catalyst depends on the size and properties of its surface. In practice, the catalyst is usually supported on a solid porous support.
The mechanism of heterogeneous catalysis is more complicated than that of homogeneous catalysis. The mechanism of heterogeneous catalysis includes five stages, all of which are reversible.
- 1. Diffusion of reactants to the surface of a solid
- 2. Physical adsorption on the active sites of the surface of a solid substance of reacting molecules and then their chemisorption
- 3. Chemical reaction between reacting molecules
- 4. Desorption of products from the catalyst surface
- 5. Diffusion of the product from the catalyst surface into the general flow
An example of heterogeneous catalysis is the oxidation of SO 2 to SO 3 on a V 2 O 5 catalyst in the production of sulfuric acid (contact method).
Most catalytic reactions are carried out on porous catalysts, the inner surface of which consists of pores and channels of various sizes and lengths. These pores may be isolated or connected to each other. The main factor determining the rate and nature of the movement of gases in the pores of the catalyst is the pore size. The speed of the free movement of molecules can reach 1000 m/s, and the deceleration of movement in the pores is associated with collisions between gas molecules and with the walls of the pores.
Most catalytic reactions are nonselective, which imposes known limitations on kinetic methods of analysis.
Most catalytic reactions involve several different types of atoms and molecules. Determining the mechanism of the reaction and the nature of the forces acting between these atoms and molecules and between them and the surface is, of course, a difficult task, but it can be simplified by studying the adsorption behavior of one type of atoms or molecules. Such studies have shown that when certain molecules are adsorbed on certain adsorbents, the bond in the molecule is broken and two bonds with the adsorbent appear; in this case, the adsorbed molecule transforms into two adsorbed atoms. This process is a surface chemical reaction, and the formed adsorbed atoms are called chemisorbed atoms. If such a reaction does not occur at sufficiently low temperatures and the adsorbed molecules do not decompose into two adsorbed atoms, then such molecules are called physically adsorbed.
acceleration of chemical reactions under the action of small amounts of substances (catalysts), which themselves do not change during the reaction. Catalytic processes play a huge role in our life. Biological catalysts called enzymes are involved in the regulation of biochemical processes. Many industrial processes would not be possible without catalysts.The most important property of catalysts is selectivity, i.e. the ability to increase the rate of only certain chemical reactions out of many possible. This allows reactions that are too slow under normal conditions to be of practical use, and ensures the formation of the desired products.
The use of catalysts contributed to the rapid development of the chemical industry. They are widely used in oil refining, obtaining various products, creating new materials (for example, plastics), often cheaper than those used before. Approximately 90% of modern chemical production is based on catalytic processes. Catalytic processes play a special role in environmental protection.
In 1835, the Swedish chemist J. Berzelius found that in the presence of certain substances, the rate of certain chemical reactions increases significantly. For such substances, he introduced the term "catalyst" (from the Greek.
catalysis- relaxation). According to Berzelius, catalysts have a special ability to weaken the bonds between atoms in the molecules involved in the reaction, thus facilitating their interaction. A great contribution to the development of ideas about the operation of catalysts was made by the German physicochemist W. Ostwald, who in 1880 defined a catalyst as a substance that changes the reaction rate.According to modern concepts, a catalyst forms a complex with reacting molecules, which is stabilized by chemical bonds. After rearrangement, this complex dissociates to release products and catalyst. For a monomolecular reaction of the transformation of a molecule
X to Y The whole process can be represented as X + Cat. ® X -Cat. ® Y -Cat. ® Y + Cat. The liberated catalyst re-binds with X , and the whole cycle is repeated many times, providing the formation of large quantities of the product - substance Y . Many substances under normal conditions do not enter into a chemical reaction with each other. So, hydrogen and carbon monoxide at room temperature do not interact with each other, since the bond between atoms in a molecule H2 strong enough and does not break when attacked by a molecule CO . Catalyst Brings Molecules Together H2 and CO by forming connections with them. After rearrangement, the catalyst-reactant complex dissociates to form a product containing atoms C, H, and O. Often, when the same substances interact, different products are formed. The catalyst can direct the process along the path most favorable for the formation of a particular product. Consider the reaction between CO and H2 . In the presence of a copper-containing catalyst, methanol is practically the only reaction product:At first, CO and H molecules 2 adsorbed on the catalyst surface. Then the CO molecules form chemical bonds with the catalyst (chemisorption occurs), remaining in the undissociated form. Hydrogen molecules are also chemisorbed on the catalyst surface, but dissociate at the same time. As a result of the rearrangement, the transition complex H-Cat.- CH2OH . After adding an atom H the complex breaks down to release CH 3 OH and catalyst. In the presence of a nickel catalyst, both CO and H 2 are chemisorbed on the surface in a dissociated form, and the Cat.-CH complex is formed 3 . The end products of the reaction are CH 4 and H 2 O: Most catalytic reactions are carried out at certain pressures and temperatures by passing the reaction mixture, which is in a gaseous or liquid state, through a reactor filled with catalyst particles. The following concepts are used to describe the reaction conditions and characterize the products. Space velocity - the volume of gas or liquid passing through a unit volume of the catalyst per unit time. Catalytic activity - the amount of reactants converted by the catalyst into products per unit of time. Conversion is the proportion of a substance converted in a given reaction. Selectivity is the ratio of the amount of a certain product to the total amount of products (usually expressed as a percentage). Yield - the ratio of the amount of a given product to the amount of starting material (usually expressed as a percentage). Productivity - the amount of reaction products formed per unit volume per unit time. TYPES OF CATALYSTS
Catalysts are classified according to the nature of the reaction they promote, their chemical composition, or their physical properties. Almost all chemical elements and substances have catalytic properties to one degree or another - by themselves or, more often, in various combinations. According to their physical properties, catalysts are divided into homogeneous and heterogeneous. Heterogeneous catalysts are solids that are homogeneous and dispersed in the same gaseous or liquid medium as the reactants.
Most catalytic reactions are carried out at certain pressures and temperatures by passing the reaction mixture, which is in a gaseous or liquid state, through a reactor filled with catalyst particles. The following concepts are used to describe the reaction conditions and characterize the products. Space velocity - the volume of gas or liquid passing through a unit volume of the catalyst per unit time. Catalytic activity - the amount of reactants converted by the catalyst into products per unit of time. Conversion is the proportion of a substance converted in a given reaction. Selectivity is the ratio of the amount of a certain product to the total amount of products (usually expressed as a percentage). Yield - the ratio of the amount of a given product to the amount of starting material (usually expressed as a percentage). Productivity - the amount of reaction products formed per unit volume per unit time. TYPES OF CATALYSTS
Catalysts are classified according to the nature of the reaction they promote, their chemical composition, or their physical properties. Almost all chemical elements and substances have catalytic properties to one degree or another - by themselves or, more often, in various combinations. According to their physical properties, catalysts are divided into homogeneous and heterogeneous. Heterogeneous catalysts are solids that are homogeneous and dispersed in the same gaseous or liquid medium as the reactants. Many heterogeneous catalysts contain metals. Some metals, especially those related to
VIII group of the periodic system of elements, have catalytic activity by themselves; a typical example is platinum. But most metals exhibit catalytic properties, being in the composition of compounds; example - alumina (aluminum oxide Al 2 O 3 ). An unusual property of many heterogeneous catalysts is their large surface area. They are penetrated by numerous pores, the total area of which sometimes reaches 500 m 2 per 1 g of catalyst. In many cases, oxides with a large surface area serve as a substrate on which metal catalyst particles are deposited in the form of small clusters. This ensures efficient interaction of the reagents in the gas or liquid phase with the catalytically active metal. A special class of heterogeneous catalysts are zeolites - crystalline minerals of the group of aluminosilicates (compounds of silicon and aluminum). Although many heterogeneous catalysts have a large surface area, they usually have only a small number of active sites, which account for a small part of the total surface area. Catalysts can lose their activity in the presence of small amounts of chemical compounds called catalyst poisons. These substances bind to active centers, blocking them. Determining the structure of active centers is the subject of intense research.Homogeneous catalysts have different chemical nature - acids (H
2 SO 4 or H 3 RO 4 ), bases (NaOH ), organic amines, metals, most often transitional ( Fe or Rh ), in the form of salts, organometallic compounds or carbonyls. Catalysts also include enzymes - protein molecules that regulate biochemical reactions. The active site of some enzymes contains a metal atom ( Zn, Cu, Fe or Mo) . Metal-containing enzymes catalyze reactions involving small molecules ( O 2 , CO 2 or N 2 ). Enzymes have very high activity and selectivity, but they work only under certain conditions, such as those in which reactions occur in living organisms. The industry often uses the so-called. immobilized enzymes. HOW CATALYSTS WORK Energy. Any chemical reaction can proceed only if the reactants overcome the energy barrier, and for this they must acquire a certain energy. As we have already said, the catalytic reaction X ® Y consists of a series of successive stages. Each one needs energy to run.E called the activation energy. The change in energy along the reaction coordinate is shown in fig. one.Consider first the non-catalytic, "thermal" path. For a reaction to take place, the potential energy of the molecules
X must exceed the energy barrierE T . The catalytic reaction consists of three stages. The first is the formation of the X-Cat complex. (chemisorption), the activation energy of which isE ads . The second stage is the X-Cat rearrangement.®Y -Cat. with activation energyE cat , and finally, the third - desorption with activation energyE des; E ads, E kat and E des much smaller E T . Since the reaction rate depends exponentially on the activation energy, the catalytic reaction proceeds much faster than the thermal one at a given temperature.A catalyst can be likened to an instructor-guide who guides climbers (reacting molecules) through a mountain range. He leads one group through the pass and then returns for the next. The path through the pass lies much lower than that which lies through the top (the thermal channel of the reaction), and the group makes the transition faster than without a conductor (catalyst). It is even possible that on their own the group would not have been able to overcome the ridge at all.
Theories of Catalysis. Three groups of theories have been proposed to explain the mechanism of catalytic reactions: geometric, electronic, and chemical. In geometric theories, the main attention is paid to the correspondence between the geometric configuration of the atoms of the active centers of the catalyst and the atoms of that part of the reacting molecules that is responsible for binding to the catalyst. Electronic theories are based on the idea that chemisorption is due to electronic interaction associated with charge transfer, i.e. these theories relate catalytic activity to the electronic properties of the catalyst. Chemical theory considers a catalyst as a chemical compound with characteristic properties that forms chemical bonds with reactants, resulting in the formation of an unstable transition complex. After the decomposition of the complex with the release of products, the catalyst returns to its original state. The latter theory is now considered the most adequate.At the molecular level, a catalytic gas phase reaction can be represented as follows. One reacting molecule binds to the active site of the catalyst, while the other interacts with it while being directly in the gas phase. An alternative mechanism is also possible: the reacting molecules are adsorbed on neighboring active sites of the catalyst and then interact with each other. Apparently, this is how most catalytic reactions proceed.
Another concept suggests that there is a relationship between the spatial arrangement of atoms on the catalyst surface and its catalytic activity. The rate of some catalytic processes, including many hydrogenation reactions, does not depend on the mutual arrangement of catalytically active atoms on the surface; the speed of others, on the contrary, changes significantly with a change in the spatial configuration of surface atoms. An example is the isomerization of neopentane to isopentane and the simultaneous cracking of the latter to isobutane and methane on the catalyst surface.
Pt-Al 2 O 3 . APPLICATION OF CATALYSIS IN INDUSTRY The rapid industrial growth that we are now experiencing would not have been possible without the development of new chemical technologies. To a large extent, this progress is determined by the widespread use of catalysts, with the help of which low-grade raw materials are converted into high-value products. Figuratively speaking, the catalyst is the philosopher's stone of the modern alchemist, only it does not turn lead into gold, but raw materials into medicines, plastics, chemical reagents, fuel, fertilizers and other useful products.Perhaps the very first catalytic process that man learned to use is fermentation. Recipes for the preparation of alcoholic beverages were known to the Sumerians as early as 3500 BC.
Cm. WINE; BEER.A significant milestone in the practical application of catalysis was the production of margarine by catalytic hydrogenation of vegetable oil. For the first time, this reaction on an industrial scale was carried out around 1900. And starting from the 1920s, one after another, catalytic methods were developed for the production of new organic materials, primarily plastics. The key point was the catalytic production of olefins, nitriles, esters, acids, etc. - "bricks" for the chemical "construction" of plastics.
The third wave of industrial use of catalytic processes occurs in the 1930s and is associated with oil refining. In terms of volume, this production soon left all others far behind. Oil refining consists of several catalytic processes: cracking, reforming, hydrosulfonation, hydrocracking, isomerization, polymerization and alkylation.
And finally, the fourth wave in the use of catalysis is related to environmental protection. The most famous achievement in this area is the creation of a catalytic converter for automobile exhaust gases. Catalytic converters, which have been installed in cars since 1975, have played a big role in improving air quality and have saved many lives in this way.
About a dozen Nobel Prizes have been awarded for work in the field of catalysis and related fields.
The practical significance of catalytic processes is evidenced by the fact that the share of nitrogen, which is part of the nitrogen-containing compounds obtained industrially, accounts for about half of all nitrogen that is part of food products. The amount of nitrogen compounds produced naturally is limited, so that the production of dietary protein depends on the amount of nitrogen applied to the soil with fertilizers. It would be impossible to feed even half of humanity without synthetic ammonia, which is produced almost exclusively by the Haber-Bosch catalytic process.
The scope of catalysts is constantly expanding. It is also important that catalysis can significantly increase the efficiency of previously developed technologies. An example is the improvement in catalytic cracking through the use of zeolites.
Hydrogenation. A large number of catalytic reactions are associated with the activation of a hydrogen atom and some other molecule, leading to their chemical interaction. This process is called hydrogenation and underlies many stages of oil refining and the production of liquid fuels from coal (the Bergius process).The production of aviation gasoline and motor fuel from coal was developed in Germany during World War II, since there are no oil fields in this country. The Bergius process is the direct addition of hydrogen to carbon. Coal is heated under pressure in the presence of hydrogen and a liquid product is obtained, which is then processed into aviation gasoline and motor fuel. Iron oxide is used as a catalyst, as well as catalysts based on tin and molybdenum. During the war, approximately 1,400 tons of liquid fuel per day were obtained at 12 German factories using the Bergius process.
Another process, Fischer - Tropsch, consists of two stages. First, the coal is gasified, i.e. carry out its reaction with water vapor and oxygen and get a mixture of hydrogen and carbon oxides. This mixture is converted into liquid fuel using catalysts containing iron or cobalt. With the end of the war, the production of synthetic fuel from coal in Germany was discontinued.
As a result of the rise in oil prices that followed the oil embargo in 1973-1974, vigorous efforts were made to develop an economically viable method for producing gasoline from coal. Thus, direct liquefaction of coal can be carried out more efficiently using a two-stage process in which the coal is first contacted with an alumina-cobalt-molybdenum catalyst at a relatively low and then at a higher temperature. The cost of such synthetic gasoline is higher than that obtained from oil.
Ammonia. One of the simplest hydrogenation processes from a chemical point of view is the synthesis of ammonia from hydrogen and nitrogen. Nitrogen is a very inert substance. To disconnect N-N its molecule requires an energy of the order of 200 kcal/ mol. However, nitrogen binds to the surface of the iron catalyst in the atomic state, and this requires only 20 kcal./ mol. Hydrogen bonds with iron even more readily. The synthesis of ammonia proceeds as follows: This example illustrates the ability of a catalyst to accelerate both the forward and reverse reactions equally, i.e. the fact that the catalyst does not change the equilibrium position of the chemical reaction.Hydrogenation of vegetable oil.
One of the most important hydrogenation reactions in practice is the incomplete hydrogenation of vegetable oils to margarine, cooking oil, and other food products. Vegetable oils are obtained from soybeans, cotton seeds and other crops. They include esters, namely triglycerides of fatty acids with varying degrees of unsaturation. Oleic acid CH 3 (CH 2) 7 CH \u003d CH (CH 2) 7 COOH has one C=C double bond, linoleic acid has two, and linolenic acid has three. The addition of hydrogen to break this bond prevents the oils from oxidizing (rancidity). This raises their melting point. The hardness of most of the products obtained depends on the degree of hydrogenation. Hydrogenation is carried out in the presence of a fine powder of nickel deposited on a substrate or Raney nickel catalyst in a highly purified hydrogen atmosphere.Dehydrogenation. Dehydrogenation is also an industrially important catalytic reaction, although the scale of its application is incomparably smaller. With its help, for example, styrene, an important monomer, is obtained. To do this, dehydrogenate ethylbenzene in the presence of a catalyst containing iron oxide; potassium and some structural stabilizer also contribute to the reaction. On an industrial scale, propane, butane and other alkanes are dehydrogenated. Dehydrogenation of butane in the presence of an alumina-chromium catalyst produces butenes and butadiene.acid catalysis.
The catalytic activity of a large class of catalysts is due to their acidic properties. According to I. Bronsted and T. Lowry, an acid is a compound capable of donating a proton. Strong acids easily donate their protons to bases. The concept of acidity was further developed in the works of G. Lewis, who defined an acid as a substance capable of accepting an electron pair from a donor substance with the formation of a covalent bond due to the socialization of this electron pair. These ideas, together with ideas about reactions that form carbenium ions, helped to understand the mechanism of various catalytic reactions, especially those involving hydrocarbons.
This example illustrates the ability of a catalyst to accelerate both the forward and reverse reactions equally, i.e. the fact that the catalyst does not change the equilibrium position of the chemical reaction.Hydrogenation of vegetable oil.
One of the most important hydrogenation reactions in practice is the incomplete hydrogenation of vegetable oils to margarine, cooking oil, and other food products. Vegetable oils are obtained from soybeans, cotton seeds and other crops. They include esters, namely triglycerides of fatty acids with varying degrees of unsaturation. Oleic acid CH 3 (CH 2) 7 CH \u003d CH (CH 2) 7 COOH has one C=C double bond, linoleic acid has two, and linolenic acid has three. The addition of hydrogen to break this bond prevents the oils from oxidizing (rancidity). This raises their melting point. The hardness of most of the products obtained depends on the degree of hydrogenation. Hydrogenation is carried out in the presence of a fine powder of nickel deposited on a substrate or Raney nickel catalyst in a highly purified hydrogen atmosphere.Dehydrogenation. Dehydrogenation is also an industrially important catalytic reaction, although the scale of its application is incomparably smaller. With its help, for example, styrene, an important monomer, is obtained. To do this, dehydrogenate ethylbenzene in the presence of a catalyst containing iron oxide; potassium and some structural stabilizer also contribute to the reaction. On an industrial scale, propane, butane and other alkanes are dehydrogenated. Dehydrogenation of butane in the presence of an alumina-chromium catalyst produces butenes and butadiene.acid catalysis.
The catalytic activity of a large class of catalysts is due to their acidic properties. According to I. Bronsted and T. Lowry, an acid is a compound capable of donating a proton. Strong acids easily donate their protons to bases. The concept of acidity was further developed in the works of G. Lewis, who defined an acid as a substance capable of accepting an electron pair from a donor substance with the formation of a covalent bond due to the socialization of this electron pair. These ideas, together with ideas about reactions that form carbenium ions, helped to understand the mechanism of various catalytic reactions, especially those involving hydrocarbons. The strength of an acid can be determined using a set of bases that change color when a proton is added. It turns out that some industrially important catalysts behave like very strong acids. These include a Friedel-Crafts catalyst such as
HCl-AlCl 2 O 3 (or HAlCl 4 ), and aluminosilicates. The strength of the acid is a very important characteristic, since it determines the rate of protonation, a key step in the process of acid catalysis.The activity of catalysts such as aluminosilicates used in oil cracking is determined by the presence of Bronsted and Lewis acids on their surface. Their structure is similar to the structure of silica (silicon dioxide), in which some of the atoms
Si 4+ replaced by atoms Al3+. The excess negative charge that arises in this case can be neutralized by the corresponding cations. If the cations are protons, then the aluminosilicate behaves like a Brønsted acid: The activity of acid catalysts is determined by their ability to react with hydrocarbons with the formation of a carbenium ion as an intermediate product. Alkylcarbenium ions contain a positively charged carbon atom bonded to three alkyl groups and/
or hydrogen atoms. They play an important role as intermediates formed in many reactions involving organic compounds. The mechanism of action of acid catalysts can be illustrated by the example of the isomerization reactionn
-butane to isobutane in the presence of HCl - AlCl 3 or Pt - Cl - Al 2 O 3 . First, a small amount of olefin C 4 H 8 attaches a positively charged hydrogen ion to an acid catalyst to form m tertiary carbenium ion. Then negatively charged hydride ion H - split off from n
-butane to form isobutane and secondary butylcarb e no d-ion. Last as a result of rearrangement becomes tertiary carb e ni ion. This chain can continue with the elimination of a hydride ion from the next moleculen- butane, etc.:
The activity of acid catalysts is determined by their ability to react with hydrocarbons with the formation of a carbenium ion as an intermediate product. Alkylcarbenium ions contain a positively charged carbon atom bonded to three alkyl groups and/
or hydrogen atoms. They play an important role as intermediates formed in many reactions involving organic compounds. The mechanism of action of acid catalysts can be illustrated by the example of the isomerization reactionn
-butane to isobutane in the presence of HCl - AlCl 3 or Pt - Cl - Al 2 O 3 . First, a small amount of olefin C 4 H 8 attaches a positively charged hydrogen ion to an acid catalyst to form m tertiary carbenium ion. Then negatively charged hydride ion H - split off from n
-butane to form isobutane and secondary butylcarb e no d-ion. Last as a result of rearrangement becomes tertiary carb e ni ion. This chain can continue with the elimination of a hydride ion from the next moleculen- butane, etc.:  essential o that tertiary carbenium ions are more stable than primary or secondary ones. As a result, they are mainly present on the catalyst surface, and therefore the main product of butane isomerization is isobutane.
essential o that tertiary carbenium ions are more stable than primary or secondary ones. As a result, they are mainly present on the catalyst surface, and therefore the main product of butane isomerization is isobutane. Acid catalysts are widely used in oil refining - cracking, alkylation, polymerization and isomerization of hydrocarbons
(see also CHEMISTRY AND METHODS OF OIL REFINING). The mechanism of action of carbenium ions, which play the role of catalysts in these processes, has been established. At the same time, they participate in a number of reactions, including the formation of small molecules by splitting large ones, the combination of molecules (olefin with olefin or olefin with isoparaffin), structural rearrangement by isomerization, the formation of paraffins and aromatic hydrocarbons by hydrogen transfer.One of the latest industrial applications of acid catalysis is the production of leaded fuels by the addition of alcohols to isobutylene or isoamylene. The addition of oxygenated compounds to gasoline reduces the concentration of carbon monoxide in the exhaust gases. Methyl-
tert -butyl ether (MTBE) with a blending octane number of 109 also makes it possible to obtain the high-octane fuel required for the operation of an automobile engine with a high compression ratio without resorting to the introduction of tetraethyl lead into gasoline. The production of fuels with octane numbers 102 and 111 is also organized.Basic catalysis. The activity of catalysts is determined by their basic properties. An old and well-known example of such catalysts is sodium hydroxide used to hydrolyze or saponify fats in the manufacture of soap, and a recent example is the catalysts used in the production of polyurethane plastics and foams. Urethane is formed by the interaction of alcohol with isocyanate, and this reaction is accelerated in the presence of basicamines. During the reaction, the base is attached to the carbon atom in the isocyanate molecule, as a result of which a negative charge appears on the nitrogen atom and its activity with respect to alcohol increases. A particularly effective catalyst is triethylenediamine. Polyurethane plastics are obtained by reacting diisocyanates with polyols (polyalcohols). When the isocyanate reacts with water, the previously formed urethane decomposes releasing CO2 . When a mixture of polyalcohols and water interacts with diisocyanates, the resulting polyurethane foam foams with gaseous CO2. Dual action catalysts. These catalysts speed up two types of reactions and give better results than passing the reactants in series through two reactors each containing only one type of catalyst. This is due to the fact that the active sites of the double-acting catalyst are very close to each other, and the intermediate product formed on one of them immediately turns into the final product on the other.Combining a hydrogen activating catalyst with a hydrocarbon isomerization promoting catalyst gives a good result. The activation of hydrogen is carried out by some metals, and the isomerization of hydrocarbons by acids. An effective dual-acting catalyst used in oil refining to convert naphtha to gasoline is finely dispersed platinum deposited on acid alumina. The conversion of naphtha components such as methylcyclopentane (MCP) to benzene increases the octane number of gasoline. First, the MCP is dehydrogenated on the platinum part of the catalyst into an olefin with the same carbon backbone; then the olefin passes to the acid part of the catalyst, where it isomerizes to cyclohexene. The latter passes to the platinum part and dehydrogenates to benzene and hydrogen.
Dual action catalysts significantly accelerate oil reforming. They are used to isomerize normal paraffins to isoparaffins. The latter, boiling at the same temperatures as gasoline fractions, are valuable because they have a higher octane number compared to straight hydrocarbons. In addition, the transformation
n -butane to isobutane is accompanied by dehydrogenation, contributing to the production of MTBE.Stereospecific polymerization. An important milestone in history catalysis came about discovery of catalytic polymerizationa-olefins with the formation stereoregular x polymer ov. To catalysts stereospecific polymerization were discovered by K. Ziegler when he tried to explain the unusual properties of the polymers he obtained. Another chemist, J. Natta, suggested that the uniqueness of Ziegler polymers is determined by their stereoregularity. X-ray diffraction experiments have shown that polymers prepared from propylene in the presence of Ziegler catalysts are highly crystalline and indeed have a stereoregular structure. To describe such ordered structures, Natta introduced the terms " isotactic ' and 'syndiotactic'. In the case where there is no order, the term "atactic" is used: A stereospecific reaction proceeds on the surface of solid catalysts containing transition metals of the groups IVA - VIII (such as Ti, V, Cr, Zr ), which are in an incompletely oxidized state, and any compound containing carbon or hydrogen, which is associated with a metal from the groups I-III . A classic example of such a catalyst is the precipitate formed during the interaction TiCl 4 and Al(C 2 H 5 ) 3 in heptane, where titanium is reduced to the trivalent state. Thisexceptionally activethe system catalyzes the polymerization of propylene at normal temperature and pressure.catalytic oxidation.
The use of catalysts to control the chemistry of oxidation processes is of great scientific and practical importance. In some cases, oxidation must be complete, for example, when neutralizing CO and hydrocarbon contaminants in car exhaust gases.More often, however, it is desirable that the oxidation be incomplete, for example in many of the processes widely used in industry for the conversion of hydrocarbons into valuable intermediates containing functional groups such as -CHO, -COOH, -C-CO, -CN. In this case, both homogeneous and heterogeneous catalysts are used. An example of a homogeneous catalyst is a transition metal complex that is used to oxidizepair
-xylene to terephthalic acid, the esters of which serve as the basis for the production of polyester fibers.Heterogeneous oxidation catalysts.
These catalysts are usually complex solid oxides. Catalytic oxidation takes place in two stages. First, the oxide oxygen is captured by a hydrocarbon molecule adsorbed on the oxide surface. The hydrocarbon is oxidized and the oxide is reduced. The reduced oxide reacts with oxygen and returns to its original state. Using a vanadium catalyst, phthalic anhydride is obtained by partial oxidation of naphthalene or butane.Ethylene production by methane dehydrodimerization.
The synthesis of ethylene through dehydrodimerization allows natural gas to be converted into more easily transportable hydrocarbons. reaction 2CH 4 + 2O 2 ® C 2 H 4 + 2H 2 O is carried out at 850 ° With using various catalysts; best results obtained with catalyst Li - MgO . Presumably, the reaction proceeds through the formation of a methyl radical by splitting off a hydrogen atom from a methane molecule. Cleavage is carried out by incompletely reduced oxygen, for example, O 2
2-
. Methyl radicals in the gas phase recombine to form an ethane molecule and are converted to ethylene during subsequent dehydrogenation. Another example of incomplete oxidation is the conversion of methanol to formaldehyde in the presence of a silver or iron-molybdenum catalyst.Zeolites. Zeolites constitute a special class of heterogeneous catalysts. These are aluminosilicates with an ordered honeycomb structure, the cell size of which is comparable to the size of many organic molecules. They are also called molecular sieves. Of greatest interest are zeolites, the pores of which are formed by rings consisting of 8–12 oxygen ions (Fig. 2). Sometimes the pores overlap, as in the ZSM-5 zeolite (Fig. 3), which is used for the highly specific conversion of methanol to gasoline fraction hydrocarbons. Gasoline contains significant amounts of aromatic hydrocarbons and therefore has a high octane number. In New Zealand, for example, one third of all gasoline consumed is obtained using this technology. Methanol is obtained from imported methane.
Catalysts that make up the group of Y-zeolites significantly increase the efficiency of catalytic cracking due primarily to their unusual acidic properties. Replacing aluminosilicates with zeolites makes it possible to increase the yield of gasoline by more than 20%.
A stereospecific reaction proceeds on the surface of solid catalysts containing transition metals of the groups IVA - VIII (such as Ti, V, Cr, Zr ), which are in an incompletely oxidized state, and any compound containing carbon or hydrogen, which is associated with a metal from the groups I-III . A classic example of such a catalyst is the precipitate formed during the interaction TiCl 4 and Al(C 2 H 5 ) 3 in heptane, where titanium is reduced to the trivalent state. Thisexceptionally activethe system catalyzes the polymerization of propylene at normal temperature and pressure.catalytic oxidation.
The use of catalysts to control the chemistry of oxidation processes is of great scientific and practical importance. In some cases, oxidation must be complete, for example, when neutralizing CO and hydrocarbon contaminants in car exhaust gases.More often, however, it is desirable that the oxidation be incomplete, for example in many of the processes widely used in industry for the conversion of hydrocarbons into valuable intermediates containing functional groups such as -CHO, -COOH, -C-CO, -CN. In this case, both homogeneous and heterogeneous catalysts are used. An example of a homogeneous catalyst is a transition metal complex that is used to oxidizepair
-xylene to terephthalic acid, the esters of which serve as the basis for the production of polyester fibers.Heterogeneous oxidation catalysts.
These catalysts are usually complex solid oxides. Catalytic oxidation takes place in two stages. First, the oxide oxygen is captured by a hydrocarbon molecule adsorbed on the oxide surface. The hydrocarbon is oxidized and the oxide is reduced. The reduced oxide reacts with oxygen and returns to its original state. Using a vanadium catalyst, phthalic anhydride is obtained by partial oxidation of naphthalene or butane.Ethylene production by methane dehydrodimerization.
The synthesis of ethylene through dehydrodimerization allows natural gas to be converted into more easily transportable hydrocarbons. reaction 2CH 4 + 2O 2 ® C 2 H 4 + 2H 2 O is carried out at 850 ° With using various catalysts; best results obtained with catalyst Li - MgO . Presumably, the reaction proceeds through the formation of a methyl radical by splitting off a hydrogen atom from a methane molecule. Cleavage is carried out by incompletely reduced oxygen, for example, O 2
2-
. Methyl radicals in the gas phase recombine to form an ethane molecule and are converted to ethylene during subsequent dehydrogenation. Another example of incomplete oxidation is the conversion of methanol to formaldehyde in the presence of a silver or iron-molybdenum catalyst.Zeolites. Zeolites constitute a special class of heterogeneous catalysts. These are aluminosilicates with an ordered honeycomb structure, the cell size of which is comparable to the size of many organic molecules. They are also called molecular sieves. Of greatest interest are zeolites, the pores of which are formed by rings consisting of 8–12 oxygen ions (Fig. 2). Sometimes the pores overlap, as in the ZSM-5 zeolite (Fig. 3), which is used for the highly specific conversion of methanol to gasoline fraction hydrocarbons. Gasoline contains significant amounts of aromatic hydrocarbons and therefore has a high octane number. In New Zealand, for example, one third of all gasoline consumed is obtained using this technology. Methanol is obtained from imported methane.
Catalysts that make up the group of Y-zeolites significantly increase the efficiency of catalytic cracking due primarily to their unusual acidic properties. Replacing aluminosilicates with zeolites makes it possible to increase the yield of gasoline by more than 20%. In addition, zeolites are selective with respect to the size of the reacting molecules. Their selectivity is due to the size of the pores through which molecules of only certain sizes and shapes can pass. This applies to both starting materials and reaction products. For example, due to steric restrictions
pair -xylene is formed more easily than more voluminousortho- and meta -isomers. The latter are "locked" in the pores of the zeolite (Fig. 4).The use of zeolites has made a real revolution in some industrial technologies - dewaxing of gas oil and machine oil, obtaining chemical intermediates for the production of plastics by alkylation of aromatic compounds, xylene isomerization, disproportionation of toluene and catalytic cracking of oil. Zeolite is especially effective here
ZSM-5. Catalysts and environmental protection. The use of catalysts to reduce air pollution began at the end 19 40s. In 1952, A. Hagen-Smith found that hydrocarbons and nitrogen oxides, which are part of exhaust gases, react to light to form oxidants (in particular, ozone), which irritate the eyes and give other undesirable effects. Around the same time, Y. Houdry developed a method for catalytic purification of exhaust gases by oxidation CO and hydrocarbons up to CO 2 and H 2 A. In 1970, the Clean Air Declaration (revised 1977, expanded 1990) was formulated requiring all new vehicles from 1975 models to be equipped with catalytic converters. Norms have been established for the composition of exhaust gases. Since lead compounds added to gasoline poison catalysts, a phase-out program has been adopted. Attention was also drawn to the need to reduce the content of nitrogen oxides.Catalysts have been created specifically for automotive converters, in which active components are deposited on a ceramic substrate with a honeycomb structure, through the cells of which exhaust gases pass. The substrate is coated with a thin layer of metal oxide, for example
Al2O3 on which a catalyst is applied - platinum, palladium or rhodium. The content of nitrogen oxides formed during the combustion of natural fuels at thermal power plants can be reduced by adding small amounts of ammonia to the flue gases and passing them through a titanium-vanadium catalyst.Enzymes. Enzymes are natural catalysts that regulate biochemical processes in a living cell. They participate in the processes of energy exchange, the breakdown of nutrients, biosynthesis reactions. Many complex organic reactions cannot proceed without them. Enzymes function at ordinary temperature and pressure, have very high selectivity and are able to increase the rate of reactions by eight orders of magnitude. Despite these advantages, only approx. Of the 15,000 known enzymes, 20 are used on a large scale.Man has been using enzymes for thousands of years to bake bread, produce alcoholic beverages, cheese and vinegar. Now enzymes are also used in industry: in the processing of sugar, in the production of synthetic antibiotics, amino acids and proteins. Proteolytic enzymes that accelerate hydrolysis processes are added to detergents.
With the help of bacteria
Clostridium acetobutylicum H. Weizmann carried out the enzymatic conversion of starch into acetone and butyl alcohol. This method of obtaining acetone was widely used in England during the First World War, and during the Second World War, butadiene rubber was made with its help in the USSR.An exceptionally large role was played by the use of enzymes produced by microorganisms for the synthesis of penicillin, as well as streptomycin and vitamin
B12. Enzymatically produced ethyl alcohol is widely used as an automotive fuel. In Brazil, more than a third of the approximately 10 million cars run on 96% ethyl alcohol derived from sugar cane, and the rest on a mixture of gasoline and ethyl alcohol (20%). The technology for the production of fuel, which is a mixture of gasoline and alcohol, is well developed in the United States. In 1987, approx. 4 billion liters of alcohol, of which approximately 3.2 billion liters were used as fuel. Various applications are also found in the so-called. immobilized enzymes. These enzymes are associated with a solid carrier, such as silica gel, over which the reagents are passed. The advantage of this method is that it ensures efficient contact of the substrates with the enzyme, separation of products and preservation of the enzyme. One example of the industrial use of immobilized enzymes is isomerization D -glucose to fructose. TECHNOLOGICAL ASPECTS Modern technologies cannot be imagined without the use of catalysts. Catalytic reactions can proceed at temperatures up to 650° C and pressures of 100 atm or more. This makes it necessary to solve the problems associated with the contact between gaseous and solid substances and with the transfer of catalyst particles in a new way. For the process to be effective, its modeling must take into account the kinetic, thermodynamic and hydrodynamic aspects. Computer modeling is widely used here, as well as new instruments and methods for controlling technological processes.In the 1960s significant progress was made in the production of ammonia. The use of a more active catalyst made it possible to lower the temperature of hydrogen production during the decomposition of water vapor, due to which it was possible to lower the pressure and, consequently, reduce production costs, for example, through the use of cheaper centrifugal compressors. As a result, the cost of ammonia fell by more than half, there was a huge increase in its production, and in connection with this - an increase in food production, since ammonia is a valuable fertilizer.
Methods. Research in the field of catalysis is carried out using both traditional and special methods. Radioactive labels, X-ray, infrared and Raman (Raman) spectroscopy, electron microscopy methods are used; kinetic measurements are carried out, the influence of the methods of obtaining catalysts on their activity is studied. Of great importance is the determination of the surface area of the catalyst by the Brunauer-Emmett-Teller method (BET method), based on the measurement of nitrogen physical adsorption at different pressures. To do this, determine the amount of nitrogen required for the formation of a monolayer on the surface of the catalyst, and, knowing the diameter of the molecule N 2 , calculate the total area. In addition to determining the total surface area, chemisorption of various molecules is carried out, which makes it possible to estimate the number of active centers and obtain information about their properties.Researchers have at their disposal various methods for studying the surface structure of catalysts at the atomic level. Unique information allows you to get a method
EXAFS . Among the spectroscopic methods, UV, X-ray, and Auger photoelectron spectroscopy are increasingly being used. Of great interest is secondary ion mass spectrometry and ion scattering spectroscopy. NMR measurements are used to study the nature of catalytic complexes. The scanning tunneling microscope allows you to see the arrangement of atoms on the surface of the catalyst. PERSPECTIVES The scale of catalytic processes in industry is increasing every year. Catalysts are increasingly being used to neutralize environmental pollutants. The role of catalysts in the production of hydrocarbons and oxygen-containing synthetic fuels from gas and coal is growing. It seems very promising to create fuel cells for the economical conversion of fuel energy into electrical energy.New concepts of catalysis will make it possible to obtain polymeric materials and other products with many valuable properties, improve energy production methods, increase food production, in particular through the synthesis of proteins from alkanes and ammonia with the help of microorganisms. It may be possible to develop genetically engineered methods for the production of enzymes and organometallic compounds that approach natural biological catalysts in their catalytic activity and selectivity.
LITERATURE Gates B.K. Chemistry of catalytic processes . M., 1981Boreskov G.K. Catalysis. Questions of theory and practice . Novosibirsk, 1987
Gankin V.Yu., Gankin Yu.V.New general theory of catalysis . L., 1991
Tokabe K. Catalysts and catalytic processes . M., 1993
S. I. LEVCHENKOV
PHYSICAL AND COLLOID CHEMISTRY
Abstract of lectures for students of the Faculty of Biology of the Southern Federal University (RSU)
2.3 CATALYTIC PROCESSES
The rate of a chemical reaction at a given temperature is determined by the rate of formation of the activated complex, which, in turn, depends on the value of the activation energy. In many chemical reactions, the structure of the activated complex may include substances that are not stoichiometrically reactants; Obviously, in this case, the value of the activation energy of the process also changes. In the case of the presence of several transition states, the reaction will proceed mainly along the path with the lowest activation barrier.
Catalysis is the phenomenon of changing the rate of a chemical reaction in the presence of substances whose state and quantity remain unchanged after the reaction.
Distinguish positive and negative catalysis (respectively, an increase and decrease in the reaction rate), although often the term "catalysis" means only positive catalysis; negative catalysis is called inhibition.
A substance that is part of the structure of an activated complex, but is not stoichiometrically a reactant, is called a catalyst. All catalysts are characterized by such general properties as specificity and selectivity of action.
Specificity The catalyst lies in its ability to accelerate only one reaction or a group of reactions of the same type and not affect the rate of other reactions. For example, many transition metals (platinum, copper, nickel, iron, etc.) are catalysts for hydrogenation processes; aluminum oxide catalyzes hydration reactions, etc.
Selectivity catalyst - the ability to accelerate one of the parallel reactions possible under given conditions. Due to this, it is possible, using different catalysts, to obtain different products from the same starting materials:
|
: CO + H 2 ––> CH 3 OH |
: C 2 H 5 OH -–> C 2 H 4 + H 2 O |
|
: CO + H 2 -–> CH 4 + H 2 O |
: C 2 H 5 OH -–> CH 3 CHO + H 2 |
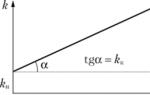
The reason for the increase in the reaction rate with positive catalysis is the decrease in the activation energy when the reaction proceeds through the activated complex with the participation of the catalyst (Fig. 2.8).
Since, according to the Arrhenius equation, the rate constant of a chemical reaction is exponentially dependent on the activation energy, a decrease in the latter causes a significant increase in the rate constant. Indeed, if we assume that the pre-exponential factors in the Arrhenius equation (II.32) for catalytic and non-catalytic reactions are close, then for the ratio of rate constants we can write:
If ΔE A = –50 kJ/mol, then the ratio of the rate constants will be 2.7·10 6 times (indeed, in practice, such a decrease in E A increases the reaction rate by approximately 10 5 times).
It should be noted that the presence of a catalyst does not affect the magnitude of the change in the thermodynamic potential as a result of the process and, therefore, no catalyst can make a thermodynamically impossible process spontaneous (of a process whose ΔG (ΔF) is greater than zero). The catalyst does not change the value of the equilibrium constant for reversible reactions; the effect of the catalyst in this case consists only in accelerating the achievement of an equilibrium state.
Depending on the phase state of the reactants and the catalyst, homogeneous and heterogeneous catalysis are distinguished.

Rice. 2.8 Energy diagram of a chemical reaction without a catalyst (1)
and in the presence of a catalyst (2).
2.3.1 Homogeneous catalysis.
Homogeneous catalysis is a catalytic reaction in which the reactants and the catalyst are in the same phase. In the case of homogeneous catalytic processes, the catalyst forms intermediate reactive products with the reagents. Consider some reaction
A + B ––> C
In the presence of a catalyst, two fast steps are carried out, resulting in the formation of particles of the intermediate compound AA and then (via the activated complex AVK #) the final reaction product with catalyst regeneration:
A + K ––> AK
AK + V -–> C + K
An example of such a process is the decomposition of acetaldehyde, the activation energy of which is E A = 190 kJ/mol:
CH 3 CHO -–> CH 4 + CO
In the presence of iodine vapor, this process proceeds in two stages:
CH 3 CHO + I 2 ––> CH 3 I + HI + CO
CH 3 I + HI -–> CH 4 + I 2
The decrease in the activation energy of this reaction in the presence of a catalyst is 54 kJ/mol; in this case, the reaction rate constant increases approximately by a factor of 105. The most common type of homogeneous catalysis is acid catalysis, in which hydrogen ions H + act as a catalyst.
2.3.2 Autocatalysis.
Autocatalysis- the process of catalytic acceleration of a chemical reaction by one of its products. An example is the hydrolysis of esters catalyzed by hydrogen ions. The acid formed during hydrolysis dissociates with the formation of protons, which accelerate the hydrolysis reaction. A feature of the autocatalytic reaction is that this reaction proceeds with a constant increase in the concentration of the catalyst. Therefore, in the initial period of the reaction, its rate increases, and at subsequent stages, as a result of a decrease in the concentration of reagents, the rate begins to decrease; the kinetic curve of the product of an autocatalytic reaction has a characteristic S-shaped form (Fig. 2.9).

Rice. 2.9 Kinetic curve of the autocatalytic reaction product
2.3.3 Heterogeneous catalysis.
heterogeneous catalysis - catalytic reactions occurring at the interface between the phases formed by the catalyst and the reactants. The mechanism of heterogeneous catalytic processes is much more complicated than in the case of homogeneous catalysis. In each heterogeneous catalytic reaction, at least six stages can be distinguished:
1. Diffusion of starting materials to the catalyst surface.
2. Adsorption of starting materials on the surface with the formation of some intermediate compound:
A + B + K -–> AVK
3. Activation of the adsorbed state (the energy required for this is the true activation energy of the process):
AVK ––> AVK #
4. Decomposition of the activated complex with the formation of adsorbed reaction products:
ABK # ––> CDK
5. Desorption of reaction products from the catalyst surface.
СDК ––> С + D + К
6. Diffusion of reaction products from the catalyst surface.
A specific feature of heterocatalytic processes is the ability of the catalyst to be promoted and poisoned.
Promotion– an increase in the activity of the catalyst in the presence of substances that are not themselves catalysts of this process (promoters). For example, for a reaction catalyzed by metallic nickel
CO + H 2 -–> CH 4 + H 2 O
the introduction of a small impurity of cerium into the nickel catalyst leads to a sharp increase in the activity of the catalyst.
Poisoning- a sharp decrease in the activity of the catalyst in the presence of certain substances (so-called catalytic poisons). For example, for the ammonia synthesis reaction (catalyst - sponge iron), the presence of oxygen or sulfur compounds in the reaction mixture causes a sharp decrease in the activity of the iron catalyst; at the same time, the ability of the catalyst to adsorb the initial substances decreases very slightly.
To explain these features of heterogeneous catalytic processes, G. Taylor made the following assumption: not the entire surface of the catalyst is catalytically active, but only some of its sections - the so-called. active centers , which may be various defects in the crystal structure of the catalyst (for example, protrusions or depressions on the surface of the catalyst). At present, there is no unified theory of heterogeneous catalysis. For metal catalysts, a multiplet theory . The main provisions of the multiplet theory are as follows:
1. The active center of the catalyst is a set of a certain number of adsorption centers located on the surface of the catalyst in geometric accordance with the structure of the molecule undergoing transformation.
2. When reacting molecules are adsorbed on the active center, a multiplet complex is formed, as a result of which the bonds are redistributed, leading to the formation of reaction products.
The theory of multiplets is sometimes called the theory of geometric similarity between the active center and reacting molecules. For different reactions, the number of adsorption centers (each of which is identified with a metal atom) in the active center is different - 2, 3, 4, etc. Such active centers are called respectively doublet, triplet, quadruplet, etc. (in the general case, a multiplet, to which the theory owes its name).
For example, according to the theory of multiplets, the dehydrogenation of saturated monohydric alcohols occurs on a doublet, and the dehydrogenation of cyclohexane - on a sextet (Fig. 2.10 - 2.11); The multiplet theory made it possible to relate the catalytic activity of metals to their atomic radius.
 Rice. 2.10 Dehydrogenation of alcohols on a doublet
Rice. 2.10 Dehydrogenation of alcohols on a doublet
 Rice. 2.11 Dehydrogenation of cyclohexane on a sextet
Rice. 2.11 Dehydrogenation of cyclohexane on a sextet
2.3.4 Enzymatic catalysis.
Enzymatic catalysis - catalytic reactions occurring with the participation of enzymes - biological catalysts of protein nature. Enzymatic catalysis has two characteristic features:
1. high activity , which is several orders of magnitude higher than the activity of inorganic catalysts, which is explained by a very significant decrease in the activation energy of the process by enzymes. So, the rate constant of the reaction of decomposition of hydrogen peroxide catalyzed by Fe 2+ ions is 56 s -1 ; the rate constant of the same reaction catalysed by the enzyme catalase is 3.5·10 7 , i.e. the reaction in the presence of the enzyme proceeds a million times faster (the activation energies of the processes are 42 and 7.1 kJ/mol, respectively). The rate constants of urea hydrolysis in the presence of acid and urease differ by thirteen orders of magnitude, amounting to 7.4·10 -7 and 5·10 6 s -1 (the activation energy is 103 and 28 kJ/mol, respectively).
2. High specificity . For example, amylase catalyzes the breakdown of starch, which is a chain of identical glucose units, but does not catalyze the hydrolysis of sucrose, the molecule of which is composed of glucose and fructose fragments.
According to generally accepted ideas about the mechanism of enzymatic catalysis, substrate S and enzyme F are in equilibrium with a very rapidly formed enzyme-substrate complex FS, which decomposes relatively slowly to the reaction product P with the release of free enzyme; thus, the stage of decomposition of the enzyme-substrate complex into reaction products is rate-determining (limiting).
F+S<––>FS ––> F+P
The study of the dependence of the rate of the enzymatic reaction on the concentration of the substrate at a constant concentration of the enzyme showed that with an increase in the concentration of the substrate, the reaction rate first increases and then ceases to change (Fig. 2.12) and the dependence of the reaction rate on the concentration of the substrate is described by the following equation:
 (II.45)
(II.45)
Introduction
1. General provisions and regularities of catalysis
2. Homogeneous catalysis
3. Acid and base catalysis
4. Homogeneous catalytic reactions catalyzed by complex compounds
5. Enzymatic catalysis
6. Heterogeneous catalysis
Conclusion
List of sources used
Introduction
Catalysis is the phenomenon of a change in the rate of a reaction in the presence of catalysts. Reactions involving catalysts are called catalytic. Substances that increase the rate of a chemical reaction, while remaining unchanged as a result of the overall reaction, are called catalysts.
There are many different types of catalysts and many different mechanisms of action. The catalyst goes through cycles in which it is first bound, then regenerated, bound again, and so on many times. The catalyst allows the reaction to proceed in a different way, and at a faster rate than it does in the absence of a catalyst. The speed can be increased by lowering the activation energy, increasing the pre-exponential factor, or both.
The catalyst simultaneously accelerates both the forward and reverse reactions, so that the equilibrium constant of the overall reaction remains unchanged. If this were not so, then it would be possible to construct a perpetual motion machine using a catalyst to regenerate matter
1. General provisions and regularities of catalysis
Catalysts are divided into homogeneous and heterogeneous. A homogeneous catalyst is in the same phase with the reactants, a heterogeneous one forms an independent phase separated by an interface from the phase in which the reactants are located. Typical homogeneous catalysts are acids and bases. Metals, their oxides and sulfides are used as heterogeneous catalysts.
Reactions of the same type can proceed with both homogeneous and heterogeneous catalysts. Thus, along with acid solutions, solid Al 2 O 3 , TiO 2 , ThO 2 , aluminosilicates, and zeolites with acidic properties are used. Heterogeneous catalysts with basic properties: CaO, BaO, MgO.
Heterogeneous catalysts, as a rule, have a highly developed surface, for which they are distributed on an inert support (silica gel, alumina, activated carbon, etc.).
For each type of reaction, only certain catalysts are effective. In addition to the acid-base ones already mentioned, there are oxidation-reduction catalysts; they are characterized by the presence of a transition metal or its compound (Co +3, V 2 O 5 +, MoO 3). In this case, catalysis is carried out by changing the oxidation state of the transition metal.
Many reactions are carried out with the help of catalysts that act through the coordination of reactants at the atom or ion of the transition metal (Ti, Rh, Ni). Such catalysis is called coordination catalysis.
If the catalyst has chiral properties, then an optically active product is obtained from an optically inactive substrate.
In modern science and technology, systems of several catalysts are often used, each of which accelerates different stages of the reaction. The catalyst can also increase the speed of one of the stages of the catalytic cycle carried out by another catalyst. This is where "catalysis of catalysis" or second-level catalysis takes place.
Enzymes play the role of catalysts in biochemical reactions.
Catalysts must be distinguished from initiators. For example, peroxides break down into free radicals that can initiate radical chain reactions. Initiators are consumed during the reaction, so they cannot be considered catalysts.
Inhibitors are sometimes mistakenly considered negative catalysts. But inhibitors, such as radical chain reactions, react with free radicals and, unlike catalysts, are not preserved. Other inhibitors (catalytic poisons) bind to the catalyst and deactivate it, which is catalysis suppression rather than negative catalysis. Negative catalysis is impossible in principle: it would provide a slower path for the reaction, but the reaction, of course, will go along a faster, in this case, not catalyzed, path.
The catalyst may be one of the reaction products. In this case, the reaction is called autocatalytic, and the phenomenon itself is called autocatalysis. For example, during the oxidation of Fe 2+ with Mn0 4
5Fe 2+ + Mn0 4 - + 8H+ \u003d 5Fe 3+ + Mn 2+ + 4H 2 0
the resulting Mn 2+ ions catalyze the course of the reaction.
Catalytic reactions are extremely common in nature. The most surprising of these are reactions with enzymes, which catalyze many reactions in living organisms. Catalysts are widely used in industry. Production of nitric and sulfuric acids, ammonia, production of synthetic rubber, etc. impossible without catalytic reactions. Catalysts are used in the production of medicinal substances: phenacetin, guaiacol, halogen derivatives of aromatic compounds, etc. Mn(IV), Ni, Co, Fe, AlC1 3 , TeC1 3 oxides are used as catalysts.
There are homogeneous and heterogeneous catalysis, but for any of them the main regularities are as follows:
1. The catalyst actively participates in the elementary act of the reaction, forming either intermediate compounds with one of the participants in the reaction, or an activated complex with all the reactants. After each elementary act, it is regenerated and can interact with new molecules of reacting substances.
2. The rate of a catalytic reaction is proportional to the amount of catalyst.
3. The catalyst has selectivity of action. It can change the rate of one reaction and not affect the rate of another.
4. The catalyst allows the reaction to proceed in a different way, and at a faster rate than it does in the absence of a catalyst.
The speed can be increased by lowering the activation energy, increasing the pre-exponential factor, or both. For example, the thermal decomposition of acetaldehyde CH 3 CHO CH 4 + CO is catalyzed by iodine vapor, which causes a decrease in the activation energy by ~55 kJ/mol. This decrease causes an increase in the rate constant by a factor of about 10,000.
5. The catalyst does not affect the position of thermodynamic equilibrium. It equally changes the rate of both forward and reverse reactions.
6. When certain substances, called promoters, are added, the activity of the catalyst increases; the addition of inhibitors reduces the rate of the reaction.
2. Homogeneous catalysis
In homogeneous catalysis, the catalyst is a molecule or ion in a homogeneous solution. In the case of homogeneous catalysis, the catalyst and all reactants form one common phase.
The main assumption of the theory of homogeneous catalysis is the idea that in the course of the reaction unstable intermediate compounds of the catalyst with the reactants are formed, which then decompose with the regeneration of the catalyst:
A + B + K = (A-B-K)* D + K
The rate of this reaction
v=k nc Ac Bc K
is proportional to the catalyst concentration, and the rate constant obeys the Arrhenius equation. This reaction can proceed in two stages:
catalysis homogeneous acid enzymatic heterogeneous
In this case, two cases are possible. In the first stage, the rate of decomposition of the complex into the catalyst and the initial product is much higher than the rate of the second stage, in which the final product is formed. Therefore, the concentration of complexes, which are called Arrhenius complexes in this type of catalysis, is low. In the second case, the rate of decomposition of the complex is commensurate with the rate of the second stage. The concentration of the intermediate complex is significant and stationary. Complexes of this type are called van't Hoff complexes.
The second case, as more typical, will be considered in more detail. Since the intermediate compound AA is in equilibrium with the starting materials, the rates of the direct (v 1) and reverse (v 2) reactions (1) must be equal. Compiling kinetic equations for them, we obtain:
where (With To"-- With AK") is the concentration of the catalyst that did not react; With A,With AK"-- equilibrium concentrations of substance A and intermediate compound AA, respectively.
From (2) we find the concentration of the intermediate compound:
The overall rate of the entire process (v) is determined by the rate of the slowest stage, in this case the second. Then
Substituting in (4) the concentration of the intermediate compound (3), we obtain:
Equation (5) indicates the possibility of the existence of two limiting regimes:
In both cases, the reaction rate is directly proportional to the concentration of the catalyst, but the reaction order for the starting materials is different. In the first case, it is equal to two, and in the second - to one. Outside the limiting regimes, the order of the reaction will be fractional.
An example of homogeneous catalysis is the reaction of thermal decomposition of acetaldehyde CH 3 CH 4 + CO, catalyzed by iodine vapor. In the absence of iodine vapor E a=191.0 kJ/mol, in their presence E a= 136.0 kJ/mol. The rate constant increases by a factor of 10,000. This is because the reaction proceeds in two stages:
CH 3 SON + I 2 \u003d CH 3 I + HI + CO
CH 3 I + HI \u003d CH 4 + I 2
The activation energy of each step is less than the activation energy of the non-catalytic reaction.
Homogeneous catalysis includes many acid-base reactions, complex formation reactions, redox reactions, numerous hydrogenation, sulfation reactions, etc.
3. Acid and base catalysis
Acids and bases in many reactions act as a catalyst, i.e., participating in the reaction, they themselves are not consumed (reactions of hydrolysis, alkylation, esterification, etc. There are three types of acid-base catalysis:
1) specific acid (basic) catalysis, in which H + or OH ions serve as a catalyst, respectively;
2) total acid (base) catalysis, which is carried out by any proton donor (acceptor);
3) electrophilic (nucleophilic) catalysis carried out by Lewis acids and bases.
First order rate constant k for the reaction in a buffer solution can be a linear function of [H + ], [OH - ], [HA], [A - ], i.e.:
k \u003d k 0 + k 1 [H+] + k 2 [OH -] + k 3 [ON] + k 4 [A -]
In this expression k 0 -- rate constant of the first order in the absence of all catalytic ions: [H + ], [OH - ], [NA], [A - ], a k t -- catalytic coefficients.
If only the term k 1 [H + ] plays a significant role, then they say that the reaction manifests itself in specific catalysis by hydrogen ions. If a member predominates k 3 [HA], the reaction is said to be subject to general acid catalysis. If the member predominates k 4 [A - ], then the reaction is said to be subject to the action of a common base catalysis.
For specific acid-base catalysis when the rate of the non-catalytic reaction is low (k 0 = 0) can be represented in logarithmic form:
For acidic solutions:
For alkaline solutions:
The equations indicate that, in the case of specific acid-base catalysis, the logarithm of the rate constant depends linearly on the pH of the medium.
The mechanism of the catalytic action of hydrogen ions is that an intermediate compound of a proton and a molecule of the original substance is formed. Due to this process, the chemical bonds present in the initial substance are loosened, the activation energy is reduced, and then the protonated form of BH + decomposes into a reaction product and a catalyst.
4. Homogeneous catalytic reactions catalyzed by complex compounds
The reactions of reduction, hydrogenation, oxidation, isomerization, polymerization under industrial conditions are carried out in the presence of catalysts - complex compounds (metal ions of group VIII of the periodic table Fe, Co, Ni, Ru, as well as Cu, Fg, Hg, Cr, Mn). The essence of the catalytic action lies in the fact that metal ions act as electron donors or acceptors. The chemical interaction between reacting molecules coordinated around the central metal ion is facilitated by polarization of the molecules and a decrease in the energy of individual bonds. The central metal ion is a bridge that facilitates electronic transitions between reacting molecules.
The catalytic activity of a metal ion depends on the binding energy of the ion with the participants in the reaction. If the binding energy is high or low, the metal ion exhibits weak catalytic activity. In the first case, the metal ions are so strongly bound to the reacting molecules that they are removed from the reaction. In the second case, the reacting molecules cannot displace other ligands present in the solution. Coordination-saturated complexes are obtained, which are not active catalysts.
Due to the wide possibilities in regulating the composition of complex catalysts, it became possible to simulate a number of reactions involving enzymes containing ions of group VIII elements.
5. Enzymatic catalysis
Enzymes are the most amazing catalysts. Many reactions in living organisms are associated with them, and therefore they are often called biological catalysts. Enzymatic catalysis is a more complex phenomenon than conventional catalysis. The high organization of enzymatic catalysis processes is determined by the peculiarity of interaction in a living organism, associated with a special combination of the molecular structure of enzymes and substrates, which are called reactants in enzymatic reactions.
Enzymes are proteins, i.e. are made up of amino acids linked by peptide bonds. The enzyme molecule has alternating polar groups COOH, NH 2 , NH, OH, SH, etc., as well as hydrophobic groups. The primary structure of an enzyme is determined by the order of alternation of the various amino acids. As a result of thermal chaotic motion, the enzyme macromolecule bends and coils into loose coils. Intermolecular interaction occurs between individual sections of the polypeptide chain, leading to the formation of hydrogen bonds. The secondary structure of the enzyme appears in the form of a loose medium. For each enzyme, the secondary structure is quite definite. The active catalytic center of the enzyme includes groups that orient the substrate molecules in a certain position. The active center is like a matrix, which can only include a molecule of a certain structure. The mechanism of enzymatic catalysis consists in the interaction of the active sites of the enzyme with the substrate to form an enzyme-substrate complex, which then undergoes several transformations, as a result of which a reaction product appears. Each of the intermediate steps is characterized by a lower activation energy, which contributes to the rapid progress of the reaction. This explains the high activity of enzymes.
Enzymes are divided into classes depending on what type of reaction they catalyze: oxidoreductases (catalyze redox reactions), transferases (catalyze the transfer of chemical groups from one compound to another), hydrolases (catalyze hydrolysis reactions), lyases (break various bonds) , isomerases (carry out isomeric transformations), ligases (catalyze synthesis reactions). As can be seen, enzymes differ in specificity and selectivity. Some catalyze a whole class of reactions of a certain type, some catalyze only one reaction.
Many enzymes contain metal ions (metal enzymes). In metalloenzymes, metal ions form chelate complexes that provide the active structure of the enzyme. Metals with a variable degree of oxidation (Fe, Mn, Cu) participate in redox reactions, carrying out the transfer of electrons to the oxidizing agent. Several dozens of organic compounds are known that perform the functions of hydrogen and electron transfer. They contain derivatives of vitamins.
Heavy metal ions (Ag + , Hg + , Pb 2+) can block the active groups of enzymes.
To assess the action of various enzymes, the concept of molecular activity was introduced, which is determined by the number of substrate molecules that are converted under the action of one enzyme molecule per minute. The most active of the known enzymes is carbonic anhydrase, whose molecular activity is ~36 million molecules per minute.
The rate of a reaction catalyzed by an enzyme is directly proportional to the concentration of the enzyme. At a low substrate concentration, the reaction is of the first order with respect to the substrate. At high concentrations, the reaction rate remains constant and the reaction order becomes zero (the enzyme is completely saturated with the substrate). The reaction rate depends on the temperature and acidity of the medium.
Enzymatic catalysis plays a huge role in all manifestations of life, where we are talking about living beings. To increase the vital activity of the body and improve metabolism, many enzyme preparations have been created that are used as medicines. Enzyme preparations are widely used for violations of the function of the gastrointestinal tract associated with insufficient production of digestive enzymes. So, in some forms of gastritis, pepsin or pancreatin preparations are used. Enzymes are also successfully used in cases where it is necessary to destroy protein formations that have accumulated in large quantities (for burns, purulent wounds, purulent-inflammatory diseases of the lungs, etc.). In these cases, protolytic enzymes are used, leading to rapid hydrolysis of proteins and facilitating the resorption of purulent accumulations. For the treatment of a number of infectious diseases, lysozyme preparations are used, which destroy the membrane of some pathogenic bacteria. Enzymes that dissolve blood clots (blood clots inside blood vessels) are very important. This is plasmin found in the blood; pancreatic enzymes - trypsin and chymotrypsin. On their basis, with various additives, medicinal enzyme preparations have been created - streptokinase, streptase, and others used in medicine.
6. Heterogeneous catalysis
Heterogeneous catalysis is carried out at the interface. The first observed heterogeneous catalytic reaction was carried out by Priestley (1778) dehydration of ethyl alcohol on active clay:
C 2 H 5 OH -- C 2 H 4 + H 2 O
In the first half of the 19th century, a large number of works were devoted to heterogeneous catalysis. Many works have been devoted to the theoretical explanation of the catalytic action of a solid. In the future, the development of the doctrine went both along the path of accumulating experimental data, developing methods for preparing catalysts, discovering and studying new catalytic processes, introducing catalysis into the chemical industry, and along the path of developing the theory of heterogeneous catalysis. However, the success of the theorists was much more modest than the success of the experimenters. And this is no coincidence.
Although there is no fundamental difference between catalytic and non-catalytic processes, both of which obey the laws of chemical kinetics, in both cases the system of reacting substances passes through some special active state, specific features are observed in heterogeneous catalytic reactions. First of all, a solid body appears, on the properties of which all phenomena as a whole essentially depend. Therefore, it is not accidental that the advances in the theory of heterogeneous catalysis are inextricably linked with the development of the theory of solids. Since the process proceeds on the surface, knowledge of the structure of the catalyst surface is decisive for the development of the theory of catalysis. From this follows a close connection between the development of the theory of catalysis and the development of the experimental and theoretical study of adsorption phenomena. The complexity of heterogeneous processes and their inherent specificity lead to the fact that theoretical research in this area has not yet been completed. So far, we can talk about the existence of several theoretical concepts that, in a first approximation, generalize certain experimental facts.
In practice, two types of heterogeneous catalysis are most often encountered:
1) processes, the catalyst of which is in the solid phase, and the reactants are in the liquid phase;
2) processes, the catalyst of which is in the solid phase, and the reactants are in the gas phase. The reaction, as a rule, occurs (and in some multistage processes begins) at the phase boundary, i.e. on the surface of a solid body - a catalyst.
The heterogeneous process can be divided into five stages:
1) transport of reactants to the catalyst surface (diffusion);
2) adsorption of reactants on the catalyst surface;
3) reaction on the surface;
4) desorption of reaction products with the release of the catalyst surface;
5) transport of reaction products into the volume (diffusion).
Depending on the conditions of the process and its characteristics, any of the five stages can be the slowest, and, consequently, the rate of the catalytic process can be limited by any of them. For a comparative assessment of the activity of catalysts, the determining factor is the reaction rate on the surface. Therefore, in those cases where it is important to obtain the value of the activity of the catalyst, they try to conduct the process in such a way that the rate is determined by the second, so-called kinetic stage.
Adsorption and desorption have their own laws. Adsorption is the process of spontaneous change in the concentration of a substance on the interface. The substance on whose surface adsorption takes place is called adsorbent. The adsorbent is called adsorbate. In heterogeneous catalysis, the adsorbent is the catalyst, and the adsorbate is the molecule of the reactant (substrate). Adsorption of the substrate on the catalyst can be carried out due to the forces of interaction arising between the molecules (atoms) of the catalyst located on the surface and the molecules of the substrate (physical adsorption). A chemical interaction (chemical adsorption or chemisorption) can occur between the molecules (atoms) of the catalyst and the molecules of the reactant. As a result of adsorption, the ordering of the system increases, the energy of the system decreases, and the activation energy of the reaction decreases.
For heterogeneous processes, the movement of a substance from the internal volume of a liquid or gas to a solid surface is of particular importance. Mass transfer processes obey the laws of diffusion.
Conclusion
The importance of catalysts and catalytic processes in oil refining and petrochemistry cannot be overestimated. After all, they are the basis of technical progress in the most important areas of meeting the needs of modern human society. The point is, first of all, that oil from various fields usually contains only 5 to 20% of light-boiling fractions corresponding to gasoline. The need for gasoline with the modern development of automobile and air transport is enormous. At the same time, motor fuels distilled directly from oil usually turn out to be of poor quality. The use of catalytic cracking and reforming in combination with other modern processing methods makes it possible to increase the yield of highly active gasolines up to 75% of the oil weight. Motor fuels are also obtained by catalytic hydrogenation of coal using metal catalysts.
Further catalytic processing of hydrocarbons on metal and oxide catalysts makes it possible to obtain intermediate products necessary in the production of consumer goods. Most monomers and polymers derived from them are products of catalytic processes for the processing of hydrocarbons and their derivatives obtained from oil, coal, shale, and natural gas. Catalytic processes play an important role in the production of detergents, dyes for medicinal substances.
The main organic synthesis giving intermediates (and products of organic technology) is based mainly on catalytic reactions. Of great importance in the life of modern society are such products of the chemical industry as sulfuric acid, ammonia and nitric acid. Almost all branches of the national economy consume these substances or other chemical compounds obtained with their help. On their basis, tens of millions of tons of mineral fertilizers are produced, without which it is impossible to increase or even maintain the yield of fields. Hundreds of industries in the chemical, petrochemical, food, light and other industries use sulfuric, nitric acids, ammonia and their derivatives. These compounds are also used in the metallurgical and metalworking industries.
Meanwhile, the large-scale production of sulfuric acid, ammonia, and nitric acid from ammonia became possible only thanks to the discovery of appropriate catalysts and the development of methods for their use.
List of sources used
1) A.P. Belyaev. Physical and colloidal chemistry. M.: GOETAR-Media, 2008
2) I.P. Mukhlenov. Catalyst technology. M.: Bookinist, 2007
3) Chemical encyclopedia. -- M.: Soviet Encyclopedia, 1990.
4) Imyanitov N.S. Systems of several catalysts in metal complex catalysis. // Coordination chemistry. 1984.
Similar Documents
Essence and features of the process of metal complex catalysis. Properties of metal complexes that determine catalytic activity. Modeling of enzymatic catalysis. Applications, advantages and disadvantages of metal complex catalysis.
report, added 03/16/2015
General theories of homogeneous catalysis. Stages of the catalysis process and reaction rate. Kinetics of the catalytic reaction of disproportionation of hydrogen peroxide in the presence of various amounts of Fe2+ catalyst, the effect of pH on the reaction rate.
test, added 09/18/2012
Determination of the rate of a chemical reaction. History of discovery, concept and types of catalytic reactions. Opinions of prominent figures of chemistry on the phenomenon of catalysis, its physical and chemical aspects. The mechanism of heterogeneous catalysis. Enzymatic catalysis in biochemistry.
abstract, added 11/14/2010
The concepts of catalysis, catalyst and catalytic process, their various definitions. Mechanisms for accelerating reactions by catalysts. Chemical (non-biological) catalysis. Synthesis of diethyl ether from alcohol with the participation of sulfuric acid. Theories of catalysis.
abstract, added 01/26/2009
Metal catalysts, mixed and polyfunctional catalysts of heterogeneous catalysis. Catalyst requirements. Theories of heterogeneous catalysis. Multiplex and electronic theory. Theory of active ensembles. Catalysis in natural gas processing.
term paper, added 05/06/2014
Definition of catalysis and its role in industry. Selectivity and general ideas about the concept of "mechanism of a chemical reaction". Classification of catalytic systems by phases and types of reactions. Adsorption and basic requirements for industrial catalysts.
abstract, added 01/26/2009
Methods for boiling arenediazonium salts. Analysis of the thermodynamic acidity of phenols. Characterization of phenol acylation, acid catalysis and phenoxyacetic acid. Features of the Kolbe-Schmitt reaction, a method for obtaining phenoxyacetic acid.
test, added 03/28/2012
The subject of thermochemistry, the study of the thermal effects of chemical reactions. Types of processes of chemical kinetics and catalysis. Enthalpy (thermal effect) of the reaction. Reaction rate, law of mass action. Chemical equilibrium constant, catalyst effect.
presentation, added 10/19/2014
Enzymes (enzymes) are biological catalysts used in the production of lactic acid products. International rules for the nomenclature of enzymes. Enzymes can only be globular proteins. Protein structure levels. Kinetics of enzymatic catalysis.
abstract, added 01/26/2009
The concept of biological catalysts, the action of enzymes in living systems and their classification. Factors affecting the activity of biological catalysts. Substances called coenzymes. Kinetics of enzymatic catalysis, Michaelis-Menten equation.